产品详情
- Cat. No.ComponentsSizeGMP-CM3102CelThera™ GMP T Cell Expansion Medium (Phenol Red-free)1000mLGMP-CM3101-1CelThera™ GMP T Cell Expansion Supplement7.25mL
产品展示(Product Show)

产品描述(Product Details)
CelThera™ GMP T Cell Expansion Medium (Phenol Red-free) is a serum-free culture medium specifically developed to support human T cell culture. It is a serum-free, animal origin-free T cell maintenance and expansion medium produced under GMP conditions.
Compared to traditional culture media or xeno-free culture media, animal origin-free culture media can better reduce the risk of introducing potential pathogenic microorganisms during culture process, improve batch-to-batch consistency, and prevent T cell overactivation by undefined components in the serum.
CelThera™ GMP T Cell Expansion Medium (Phenol Red-free) does not require the addition of any serum or serum replacements and also maintains the high fold expansion of T cells. If users choose to add serum or serum replacement, the dosage should be determined by specific T cell applications.
优势特色(Features)
- Serum-free, animal origin-free (AOF), and exogenous growth factors free.
- Designed to support low-density seeding and high fold expansion of T cells.
- Suitable for large-scale T cell expansion.
- No additional serum or serum replacements needed.
- T cell phenotypes similar to media supplemented with serum or serum replacement.
- Contains only recombinant proteins as components, and no antibiotics included in formulation.
- Produced according to current GMP guidelines.
存储(Storage)
The CelThera™ GMP T Cell Expansion Medium (Phenol Red-free) is stable for 18 months when stored under 2-8°C, protect from light.
The CelThera™ GMP T Cell Expansion Supplement is stable for 12 months when stored under -20°C or below, protect from light.质量管理控制体系(QMS)
数据展示
应用数据(Application Data)
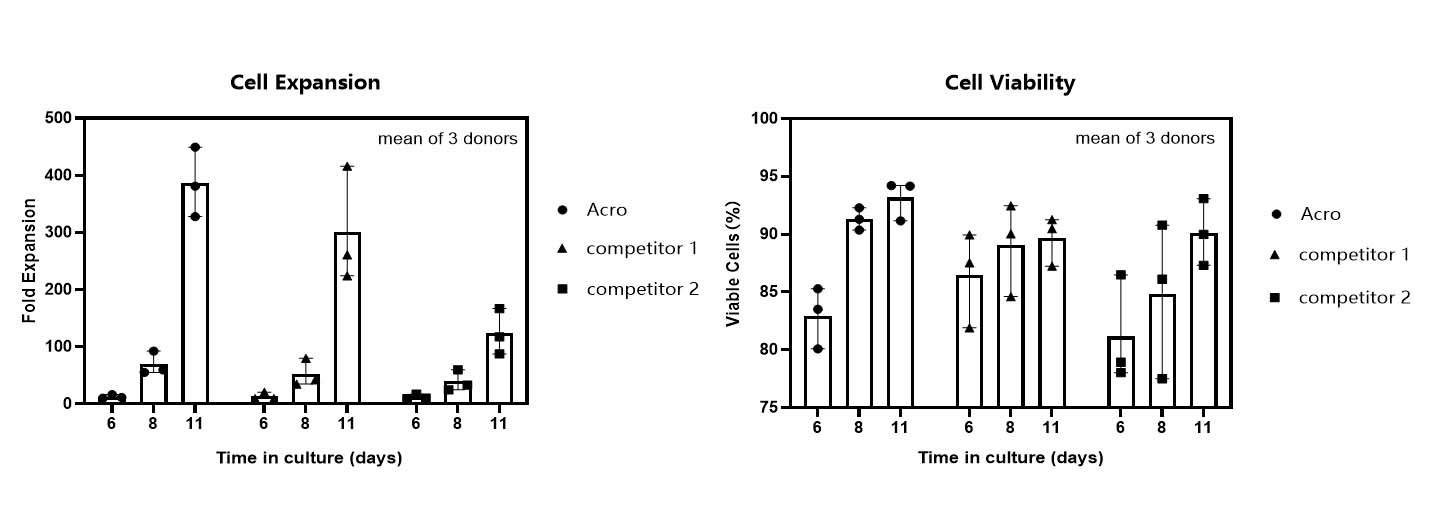
T cell expansion rate and cell viability in various media.
T cells from PBMCs of 3 different donors were activated and cultured for 11 days in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Cell count and viability were performed on day 6, day 8 and day 11 by trypan blue staining. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3102) had a faster proliferation rate and higher viability than that of the other two media.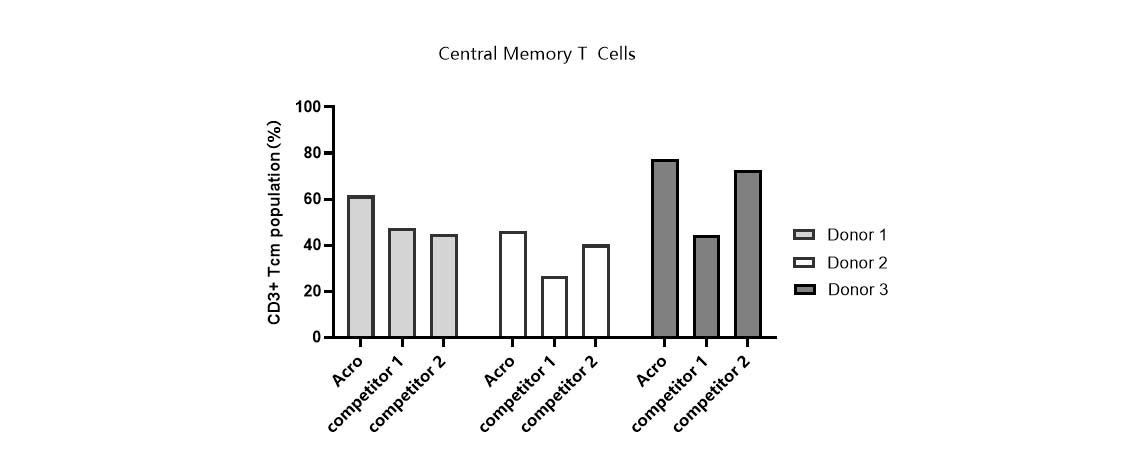
Tcm ratio in various media.
T cells from PBMCs of 3 different donors were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Tcm percentage (CD45RO+/CCR7+) was determined by flow cytometry when cells reached about 50-fold expansion. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3102) had a higher percentage of central memory T cells than that of the other two media.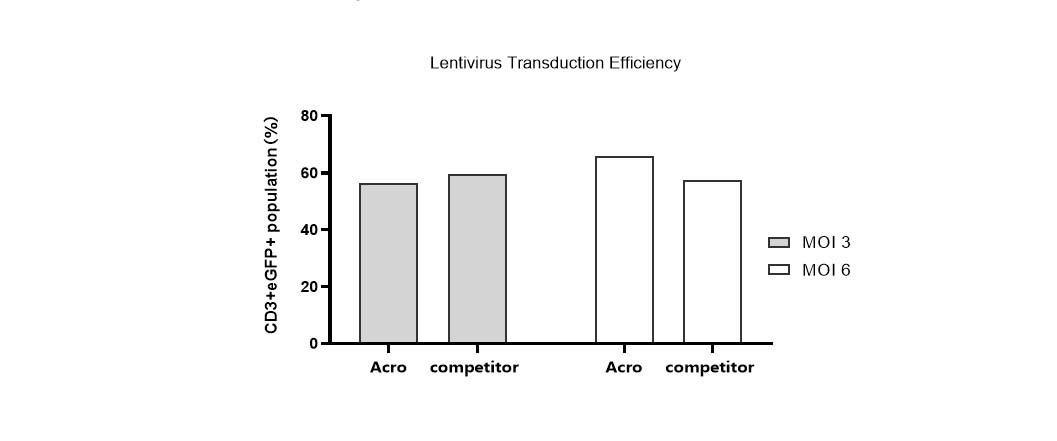
Lentivirus transduction efficiency in various media.
T cells from PBMCs were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). 24 hours after activation, the cells were transduced with pLenti-CMV-EGFP-puro lentivirus (MOI=3 or 6). 24hrs after transduction, the lentivirus was removed by centrifugation. Then, the cells were cultured for 48hrs and the CD3+eGFP+ population was detected by flow cytometry. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3102) had a similar lentiviral transduction efficiency to that of the other medium.
多供体验证数据(Multiple Donor Verification)
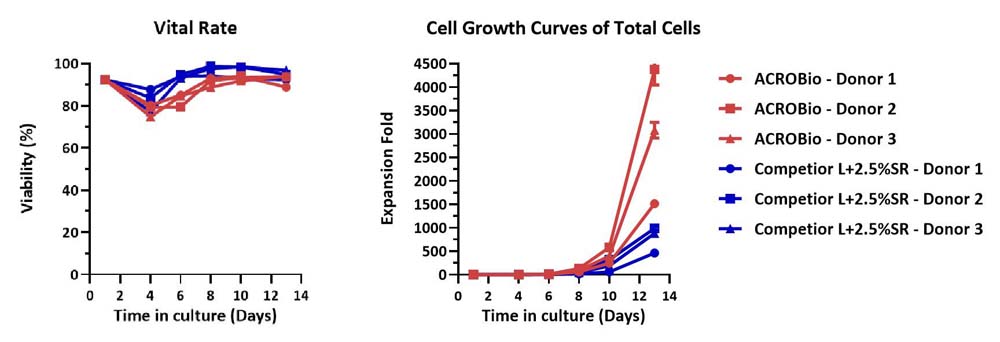
Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3102) or T cell culture medium (Competitor L +2.5% SR) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium (ACROBiosystems) can be comparable to Competitor L +2.5% SR. Notably, the cells exhibit better expansion in CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3102).
大规模培养数据(Large-scale Culture Verification)
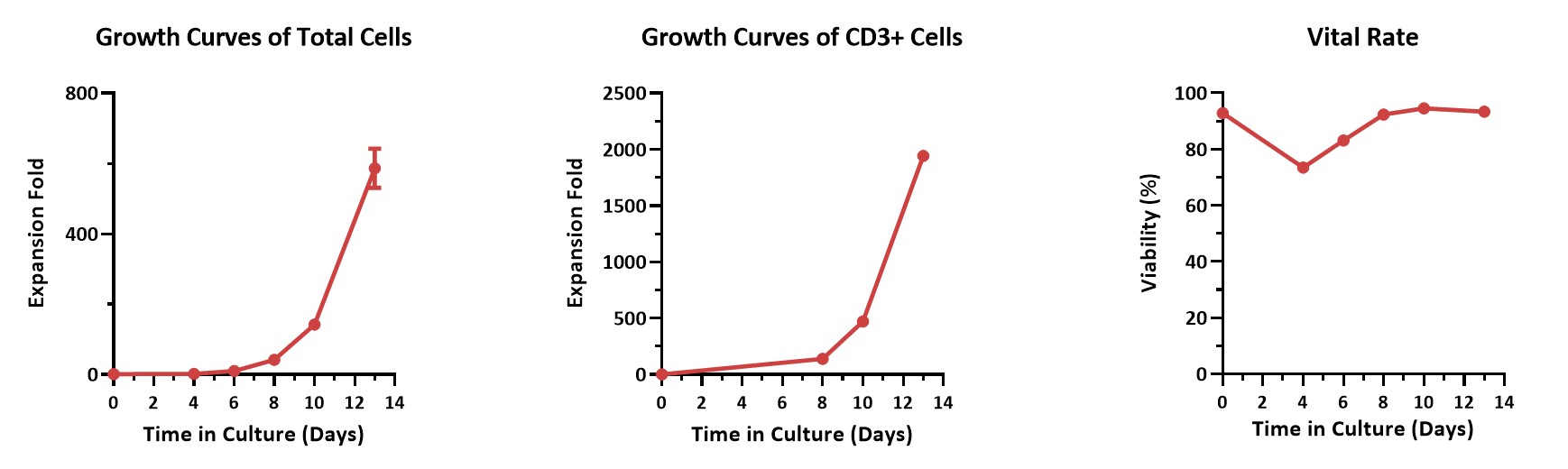
Human PBMCs were activated using 0.2 µg/mL GMP Monoclonal Anti-Human CD3 Antibody (OKT3) (ACROBiosystems, Cat. No. GMP-MC0323) and 1 µg/mL GMP Monoclonal Anti-Human CD28 Antibody (ACROBiosystems, Cat. No. GMP-MC2824), cultured with CelThera™ GMP T Cell Expansion culture medium (ACROBiosystems, Cat. No. GMP-CM3102) supplemented with 500 IU/mL GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for two weeks. The results showed that GMP human IL-2 protein, GMP monoclonal anti-human CD3 antibody (OKT3), GMP monoclonal anti-human CD28 antibody, and CelThera™ GMP T cell expansion medium could be used to culture T cells in a 3L large system. It can efficiently expand cells with high viability.
用户评价 发表评论

重要声明
MANUFACTURING SPECIFICATIONS
ACROBiosystems GMP grade mediums are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO 20399: 2022(E), Biotechnology ? Ancillary materials present during the production of cellular therapeutic products and gene therapy products.
ACROBiosystems Quality Management System Contents:- Designed and Manufactured under ISO 9001:2015 and ISO 13485:2016.
- Animal-Free materials
- Materials purchased from the approved suppliers by QA
- ISO 5 clean room for filling
- Qualified personnel
- Quality-related documents review and approve by QA
- Fully batch production and control records
- Equipment maintenance and calibration
- Validation of analytical procedures
- Stability studies conducted
- Comprehensive regulatory support files
Request For Regulatory Support Files(RSF) Request For DMF
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
- pH
- Sterility
- Osmolality
- Endotoxin Level
- Functionality
- Mycoplasma testing
- Batch-to-batch consistency
DISCLAIMER
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for direct human use.
TERMS AND CONDITIONS
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCHASE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
END USER TERMS OF USE OF PRODUCT
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.














