分子别名(Synonym)
CD4,CD4mut,LEU3
表达区间及表达系统(Source)
Biotinylated Human CD4, Fc,Avitag (CD4-H82F3) is expressed from human 293 cells (HEK293). It contains AA Lys 26 - Pro 396 (Accession # P01730-1).
Predicted N-terminus: Lys 26
Request for sequence
蛋白结构(Molecular Characterization)
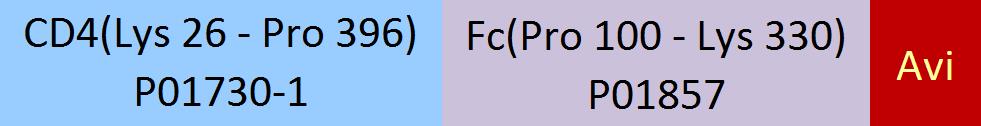
This protein carries a human IgG1 Fc tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 69.7 kDa. The protein migrates as 90-100 kDa under reducing (R) condition, and > 180 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under non-reducing (NR) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 0.2 EU per μg by the LAL method.
纯度(Purity)
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in Tris with Glycine, Arginine and NaCl, pH7.5 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

Biotinylated Human CD4, Fc,Avitag on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
SEC-MALS
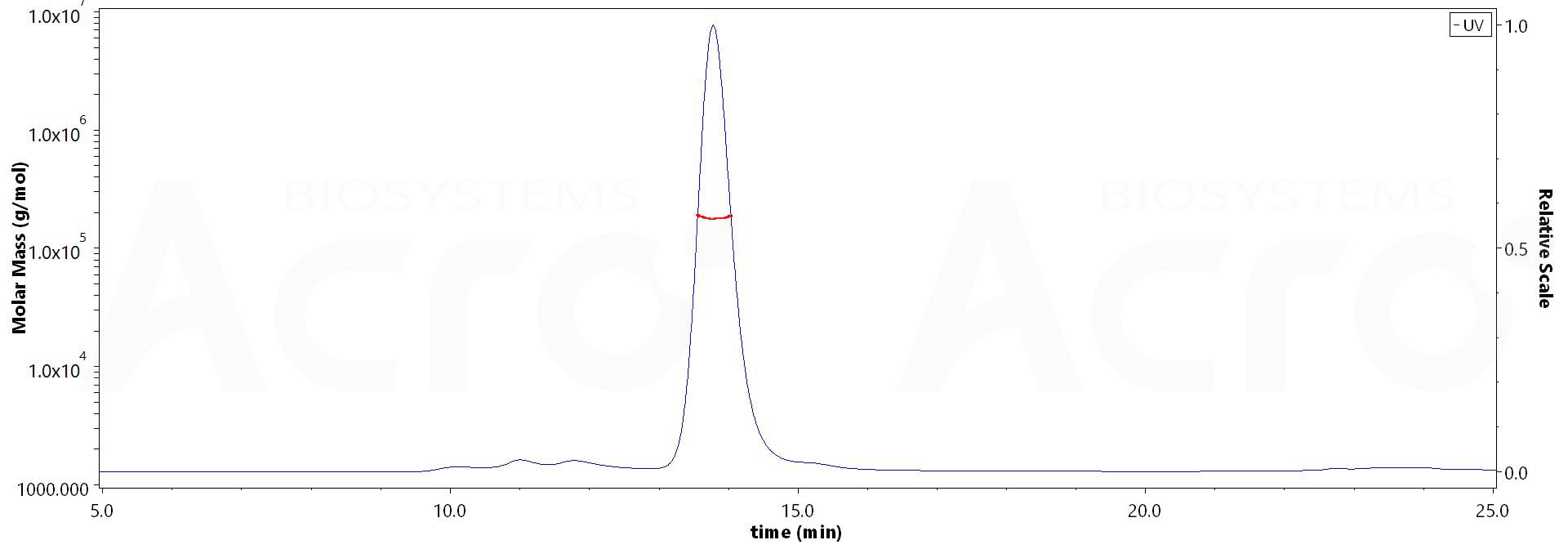
The purity of Biotinylated Human CD4, Fc,Avitag (Cat. No. CD4-H82F3) is more than 90% and the molecular weight of this protein is around 170-200 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA
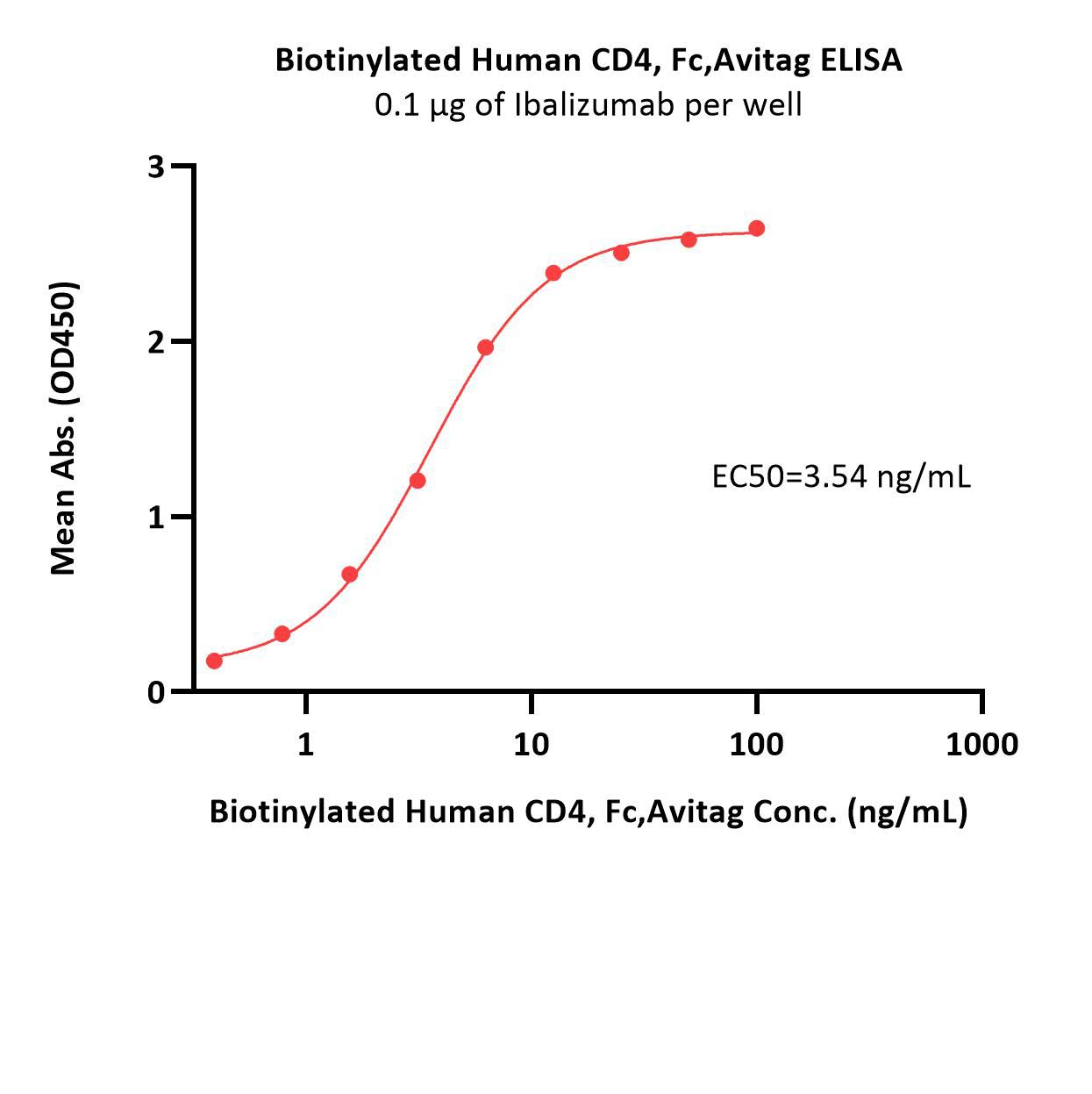
Immobilized Ibalizumab at 1 μg/mL (100 μL/well) can bind Biotinylated Human CD4, Fc,Avitag (Cat. No. CD4-H82F3) with a linear range of 0.2-6 ng/mL (QC tested).
Protocol
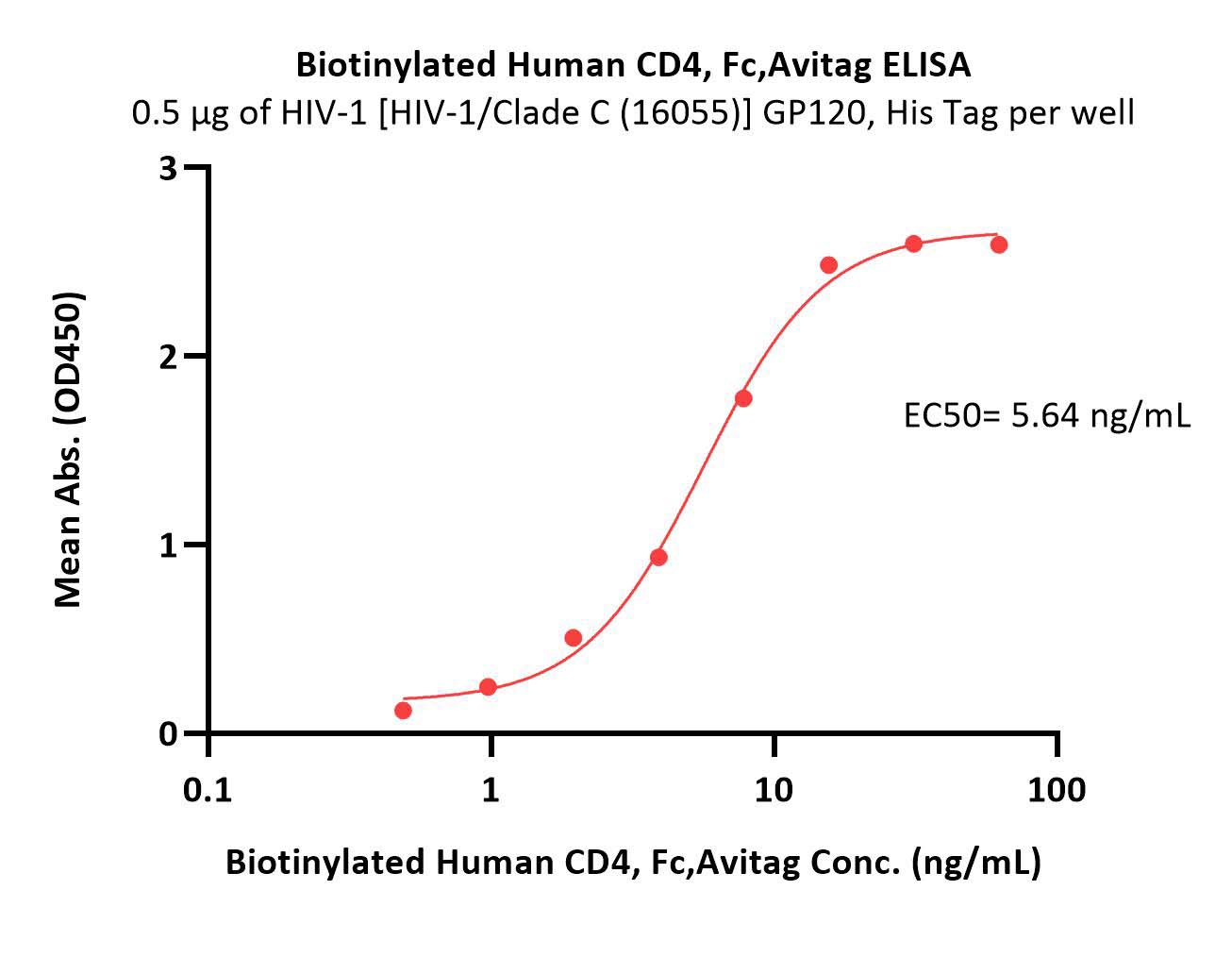
Immobilized HIV-1 [HIV-1/Clade C (16055)] GP120, His Tag (Cat. No. GP5-V15224) at 5 μg/mL (100 μL/well) can bind Biotinylated Human CD4, Fc,Avitag (Cat. No. CD4-H82F3) with a linear range of 0.5-8 ng/mL (Routinely tested).
Protocol
活性(Bioactivity)-FACS
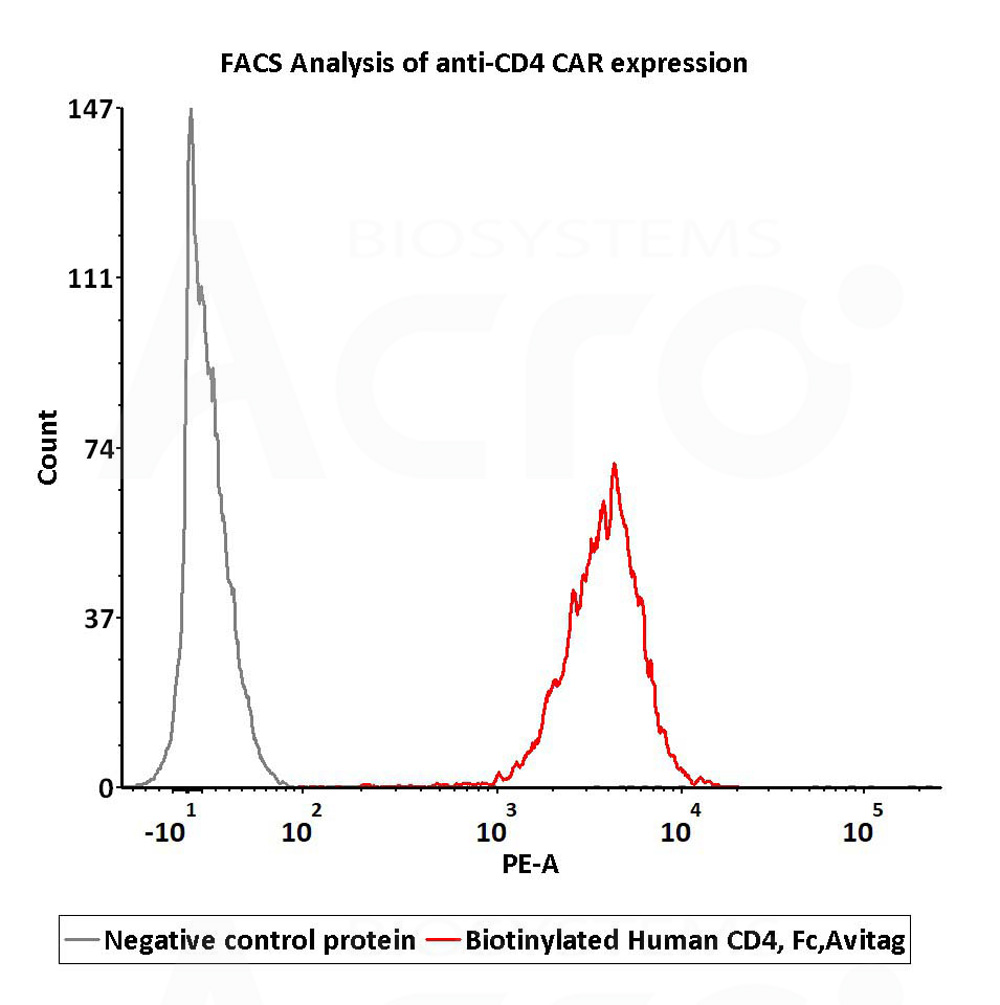
2e5 of anti-CD4 CAR-293 cells were stained with 100 μL of 1 μg/mL of Biotinylated Human CD4, Fc,Avitag (Cat. No. CD4-H82F3) and negative control protein respectively, washed and then followed by PE-SA and analyzed with FACS (Routinely tested).
Protocol
背景(Background)
T-cell surface glycoprotein CD4 is also known as T-cell surface antigen T4/Leu-3. CD4 contains three Ig-like C2-type (immunoglobulin-like) domains and one Ig-like V-type (immunoglobulin-like) domain. CD4 is accessory protein for MHC class-II antigen/T-cell receptor interaction. CD4 induces the aggregation of lipid rafts. CD4 is a primary receptor used by HIV-1 to gain entry into host T cells. HIV infection leads to a progressive reduction of the number of T cells possessing CD4 receptors. Therefore, medical professionals refer to the CD4 count to decide when to begin treatment for HIV-infected patients.























































 膜杰作
膜杰作 Star Staining
Star Staining