- Genetically modified cell lines best reflect MOA (Mechanism of Action)
- Higher activity and larger assay window for robust and reproducible cell-based bioassay
- Comprehensive application data to support assay development and validation
- Full tracible record, stringent quality control and validated cell passage stability
- Parental cell line legally obtained from internationally recognized cell resource bank and commercially licensed
- Global commercial license assistance whenever regulatory filing is required
描述(Description)
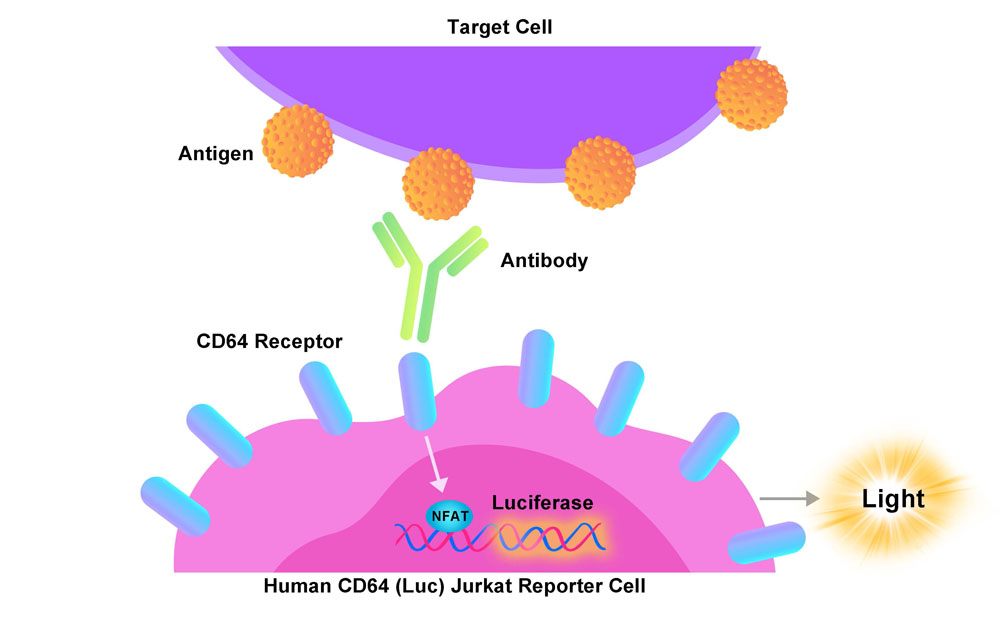
The Human CD64 (Luc) Jurkat Reporter Cell was engineered to not only express the NFAT response element driving luciferase expressing systems, but also express the receptor human CD64 (Gene ID: 2209), which can use to evaluate ADCP activity of antibodies in the presence of corresponding target cells. When co-cultured with a target cell and relevant antibody, the antibody simultaneously binds the target cell antigen and CD64 receptor on the surface of Human CD64 (Luc) Jurkat Reporter Cell, resulting in receptor clustering, intracellular signaling and NFAT-mediated luminescence.
应用说明(Application)
• Determination of ADCP activity induced by antibodies
生长特性(Growth Properties)
Suspension
筛选标记(Selection Marker)
Puromycin (5 μg/mL) + Hygromycin (20 μg/mL)
培养基(Complete Growth Medium)
RPMI-1640 + 10% FBS
冻存液(Freeze Medium)
Serum-free cell cryopreservation medium
装量(Quantity)
1 vial contains at least 5×10^6 cells in 1 mL serum-free cryopreservation medium
存储(Storage)
Frozen in liquid nitrogen.
支原体检测(Mycoplasma Testing)
Negative
无菌检测(Sterility Testing)
Negative
使用说明(Instructions for Use)
See data sheet for detailed culturing and assay protocol.
质量管理控制体系(QMS)
Receptor Assay
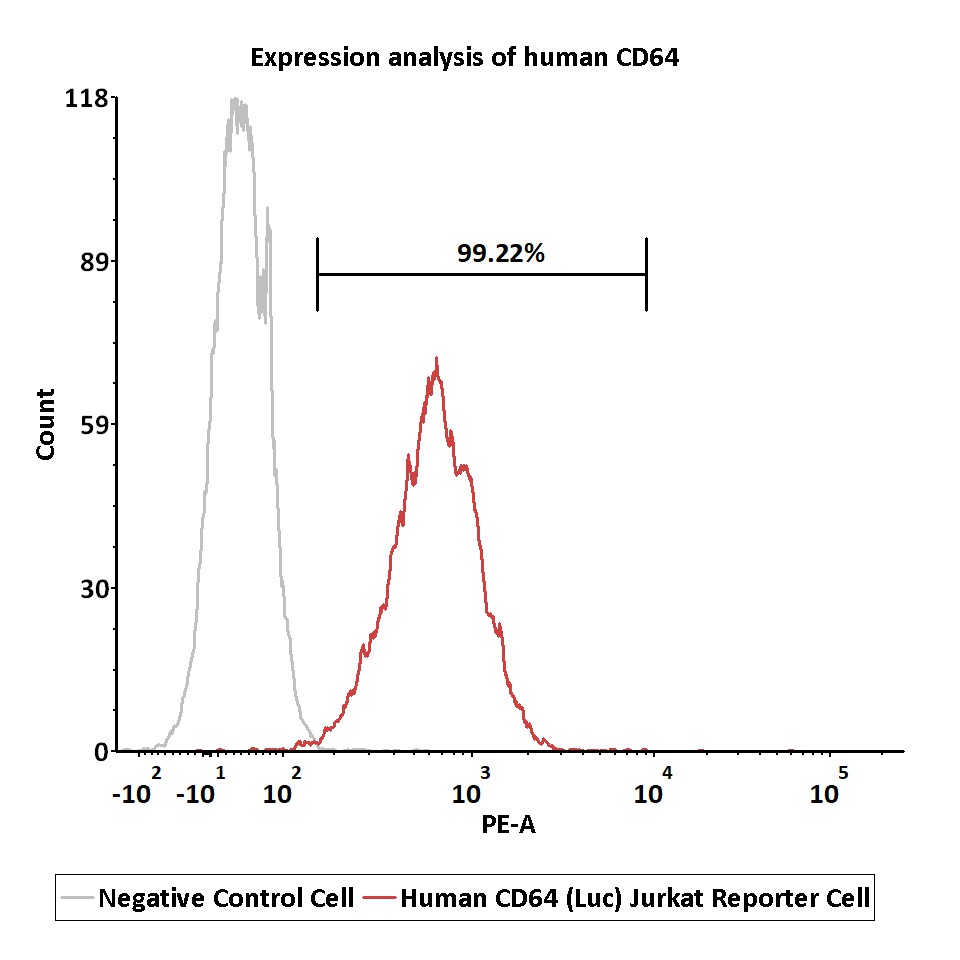
Expression analysis of human CD64 on Human CD64 (Luc) Jurkat Reporter Cell by FACS.
Human CD64 (Luc) Jurkat Reporter Cell or negative control cell were stained with PE-labeled anti-human CD64 antibody.
Protocol
Application
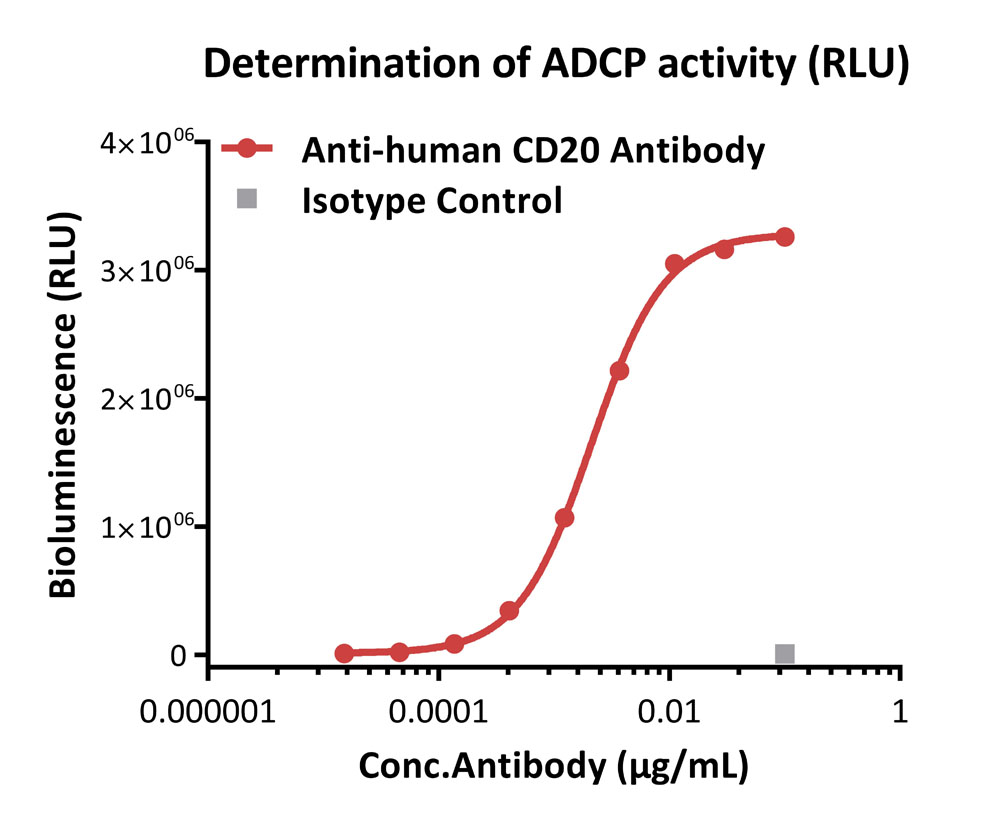
ADCP response to anti-human CD20 antibody (RLU).
Anti-human CD20 antibody-induced ADCP activity was evaluated using Human CD64 (Luc) Jurkat Reporter Cell in the presence of Raji cells that express CD20 endogenously. The EC50 of anti-human CD20 antibody was approximately 0.0021 μg/mL.
Protocol
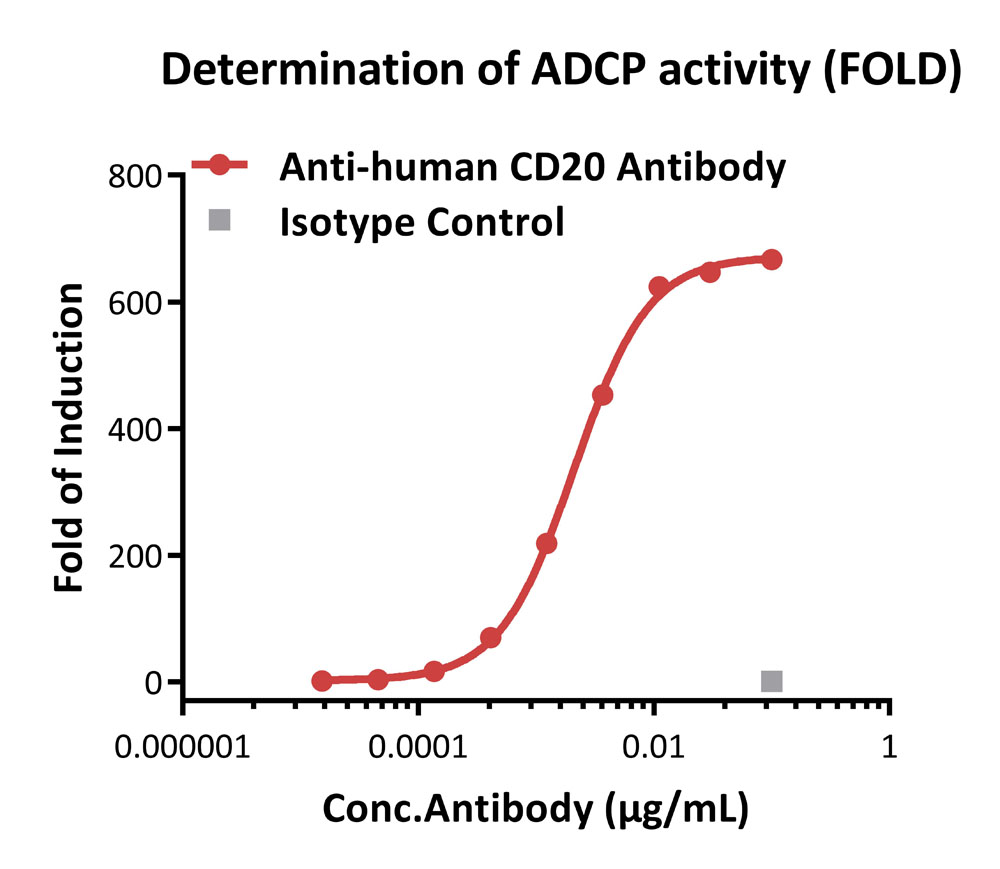
ADCP response to anti-human CD20 antibody (FOLD).
Anti-human CD20 antibody-induced ADCP activity was evaluated using Human CD64 (Luc) Jurkat Reporter Cell in the presence of Raji cells that express CD20 endogenously. The max induction fold was approximately 667.
Protocol
Passage Stability
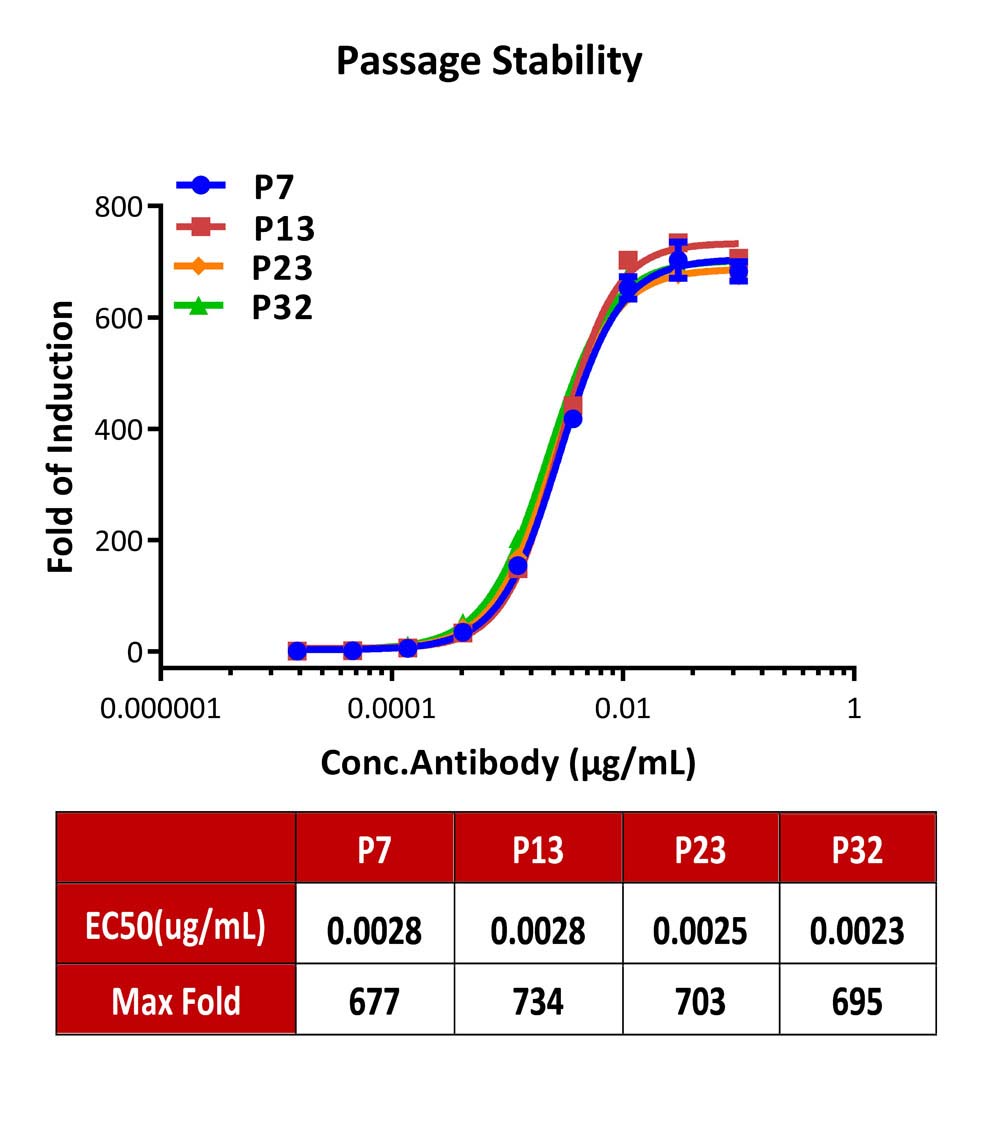
Passage stability analysis by Signaling Bioassay.
The continuously growing Human CD64 (Luc) Jurkat Reporter Cell was stimulated with serial dilutions of anti-human CD20 antibody in the presence of Raji cells that express CD20 endogenously. Anti-human CD20 antibody stimulated response demonstrates passage stabilization (fold induction and EC50) across passage 7-32.
Protocol
如有相关细胞池需求请联系我们
背景(Background)
Fcγ receptors (FcγRs), the largest group of FcRs, bind IgG and comprise several subtypes. FcγRI (CD64), a high-affinity receptor that binds monomeric uncomplexed IgG molecules. FcγRI saturation by endogenous circulating IgGs in vivo may attenuate its role in mediating antibody function.
Limited Use&License Disclosure
BY USE OF THIS PRODUCT, RESEARCHER AGREES TO BE BOUND BY THE FOLLOWING TERMS OF LIMITED USE OF THIS CELL LINE PRODUCT.
- If the researcher is not willing to accept the terms of limited use of this cell line product, and the product is unused, ACRO will accept return of the unused product.
- Researchers may use this product for research use only, no commercial use is allowed. "Commercial use" means any and all uses of this product and derivatives by a party for profit or other consideration and may include but is not limited to use in: (1) product manufacture; and (2) to provide a service, information or data; and/or resale of the product or its derivatives, whether or not such product or derivatives are resold for use in research.
- This cell line is neither intended for any animal or human therapeutic purposes nor for any direct human in vivo use . You have no right to share, modify, transfer, distribute, sell, sublicense, or otherwise make the cell line available for use to other researchers, laboratories, research institutions, hospitals, universities, or service organizations.
- ACROBIOSYSTEMS MAKES NO WARRANTIES OR REPRESENTATIONS OF ANY KIND, EITHER EXPRESSED OR IMPLIED, WITH RESPECT TO THE SUITABILITY OF THE CELL LINE FOR ANY PARTICULAR USE.
- ACROBIOSYSTEMS ACCEPTS NO LIABILITY IN CONNECTION WITH THE HANDLING OR USE OF THE CELL LINE.
- Modifications of the cell line, transfer to a third party, or commercial use of the cell line may require a separate license and additional fees. Please contact order.cn@acrobiosystems.com for further details.























































 膜杰作
膜杰作 Star Staining
Star Staining