分子别名(Synonym)
Envelope glycoprotein gp41/gp41 (HIV)
表达区间及表达系统(Source)
HIV-1 (HXB2) GP41 Pre-hairpin intermediate Protein, His Tag (GP1-H51H3) is expressed from E. coli cells. It contains AA Ala 533 - Leu 856 (Accession # P04578).
Predicted N-terminus: Met
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 44.3 kDa. The protein migrates as Band dispersion when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE).
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>85% as determined by SDS-PAGE.
制剂(Formulation)
Supplied as 0.2 μm filtered solution in 50 mM HEPES, 150 mM NaCl, CHS, pH7.5 with glycerol as protectant.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with dry ice, please inquire the shipping cost.
存储(Storage)
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- The product MUST be stored at -70°C or lower upon receipt;
- -70°C for 3 months under sterile conditions.
电泳(SDS-PAGE)

HIV-1 (HXB2) GP41 Pre-hairpin intermediate Protein, His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 85% (With Star Ribbon Pre-stained Protein Marker).
活性(Bioactivity)-ELISA
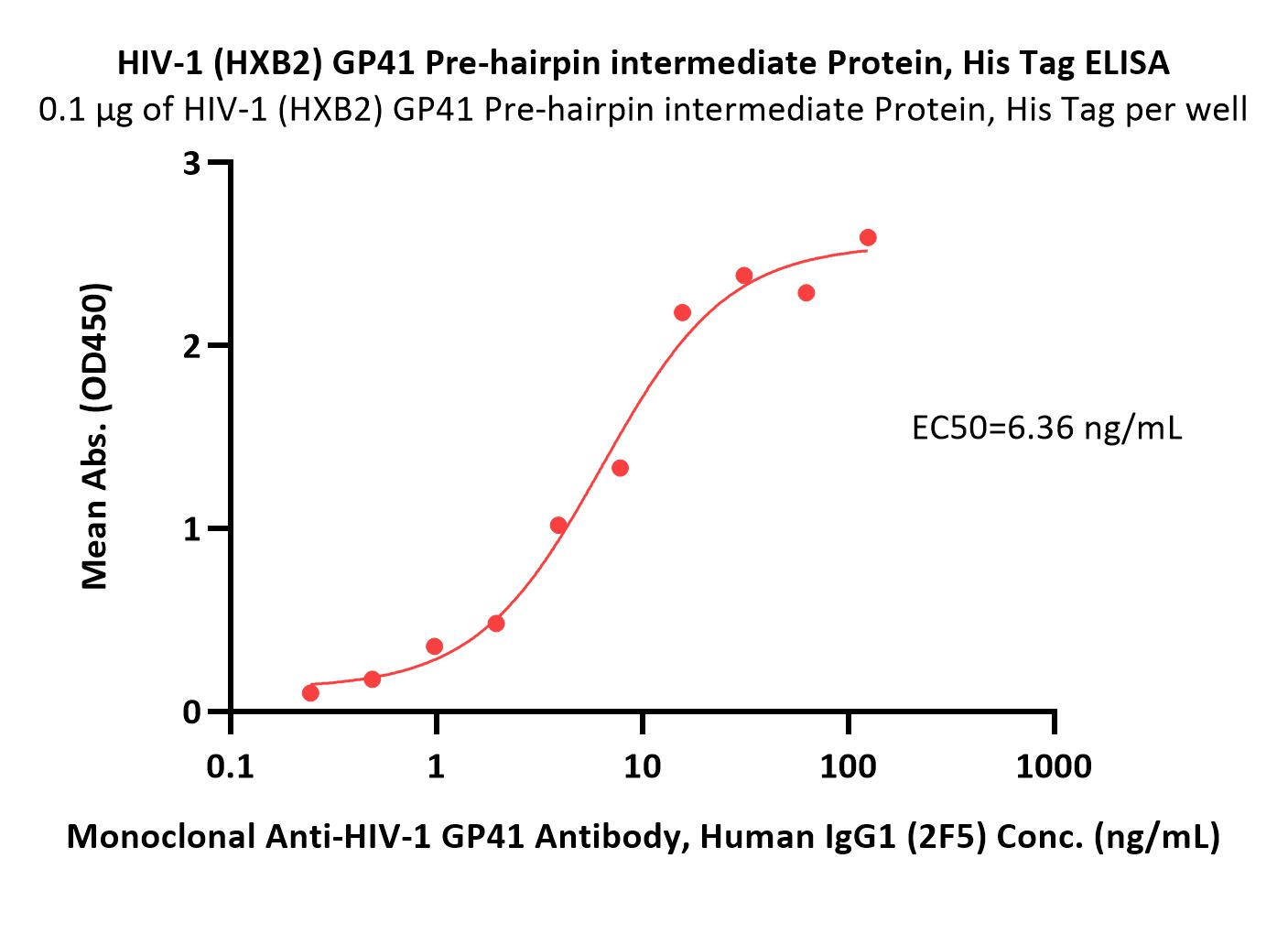
Immobilized HIV-1 (HXB2) GP41 Pre-hairpin intermediate Protein, His Tag (Cat. No. GP1-H51H3) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-HIV-1 GP41 Antibody, Human IgG1 (2F5) with a linear range of 0.2-16 ng/mL (QC tested).
Protocol
活性(Bioactivity)-SPR
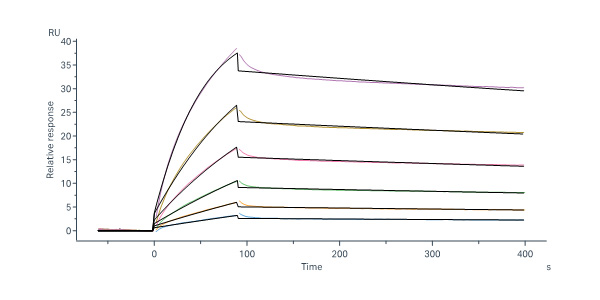
Monoclonal Anti-HIV-1 GP41 Antibody, Human IgG1 (2F5) captured on Protein A Chip can bind HIV-1 (HXB2) GP41 Pre-hairpin intermediate Protein, His Tag (Cat. No. GP1-H51H3) with an affinity constant of 0.468 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
Protocol
背景(Background)
Infection by HIV-1 involves the fusion of viral and cellular membranes with subsequent transfer of viral genetic material into the cell. The HIV-1 envelope glycoprotein that mediates fusion consists of the surface subunit gp120 and the transmembrane subunit gp41. gp120 directs virion attachment to the cell-surface receptors, and gp41 then promotes viral-cell membrane fusion. A soluble, alpha-helical, trimeric complex within gp41 composed of N-terminal and C-terminal extraviral segments has been proposed to represent the core of th e fusion-active conformation of the HIV-1 envelope. Three N-terminal helices within the bundle form a central, parallel, trimeric coiled coil, whereas three C-terminal helices pack in the reverse direction into three hydrophobic grooves on the surface of the N-terminal trimer. This thermostable subdomain displays the salient features of the core structure of the isolated gp41 subunit and thus provides a possible target for therapeutics designed selectively to block HIV-1 entry.























































 膜杰作
膜杰作 Star Staining
Star Staining