Bacterial Vaginosis Toxins Impair Sperm Capacitation and FertilizationBhagwat, Asadi, McCarthy
et albioRxiv (2025)
Abstract: What effect do toxins produced by bacterial vaginosis (BV) bacteria have on sperm function?Bacterial vaginosis toxins dysregulate sperm capacitation and intracellular calcium homeostasis and impair the ability of sperm to fertilize oocytes.In bacterial vaginosis, which is linked to infertility, overgrowth of Prevotella and Gardnerella in the vagina is accompanied by elevated concentrations of the toxins lipopolysaccharide (LPS) and vaginolysin (VLY).This was a laboratory study in which human semen samples were collected from consenting healthy donors with normal semen parameters. Mouse sperm samples were obtained from the caudal epididymis.Motile mouse and human sperm were isolated via swim-up and treated under non-capacitating or capacitating conditions. LPS from Escherichia coli was commercially available. VLY was produced by cloning the Gardnerella VLY protein in the ClearColi expression system. Mouse sperm were pre-incubated in in vitro fertilization medium with LPS or VLY and then co-cultured with ovulated cumulus-oocyte complexes. The effects of LPS and VLY on sperm motility and hyperactivation were assessed with computer-assisted sperm analysis. Effects on viability were assessed by Hoechst staining. Acrosomal exocytosis was assessed in sperm from transgenic Acr-eGFP mice and in human sperm stained with Pisum sativum agglutinin FITC. Intracellular calcium dynamics were assessed by staining sperm with the calcium-sensitive dye Fluo-4 AM and fluorescent imaging several sperm at the single-cell level. The effects of LPS on sperm from CatSper knock-out mice were assessed. Additionally, sperm were treated with a toll-like receptor 4 antagonist and further exposed to LPS.Exposure of mouse sperm to LPS or VLY significantly decreased in vitro fertilization ( P < 0.05). Under capacitating conditions, both toxins initially increased mouse and human sperm hyperactivation, then significantly decreased sperm motility ( P < 0.05), hyperactivation ( P < 0.05), and acrosomal exocytosis ( P < 0.01). These changes were accompanied by a rapid and irreversible increase in intracellular calcium concentration. Effects of LPS, but not VLY, were prevented by polymyxin-B, which aggregates LPS. The LPS-induced intracellular calcium increase required external calcium but not the calcium channel CatSper and was inhibited by the Toll-like receptor 4 antagonist.First, the commercially available LPS we used was isolated from Escherichia coli , rather than from the BV-associated bacteria Prevotella bivia . Second, we did not quantify the absolute sperm intracellular calcium concentration before or after LPS or VLY treatment. Third, all of our experiments were in vitro .These studies suggest that BV-associated toxins contribute to infertility by, in part, impairing sperm capacitation and reducing their fertilizing ability.This work was supported by the National Institutes of Health (grant #R01 HD069631). The authors declare that they have no conflict of interest.
Development of skin corrosion and irritation test methods employing ex vivo porcine skin modelHong, Lim
Toxicol In Vitro (2025)
Abstract: Skin corrosion and irritation are key local toxicological responses of the skin to chemical exposure. Conventional in vivo methods have limitations such as species difference, ethical concern, and reproducibility issue, necessitating the development of reliable alternatives. We developed skin corrosion (SCT) and irritation test (SIT) methods using ex vivo porcine ear skin based on cell viability and skin barrier disruption. Test chemicals were treated on ex vivo porcine ear skin for 3 min and 60 min (SCT) or 60 min only (SIT). After 40 ± 2 h post-incubation, cell viability assay with CCK-8, and skin barrier test with FITC-dextran penetration were conducted. To evaluate the predictive capacity of the method, 38 reference chemicals (20 corrosives, 8 irritants and 10 no category chemicals) were tested. SCT achieved 96.7 % (29/30) accuracy in identifying corrosives, meeting the performance standards of OECD TG 431 In Vitro Skin Corrosion: Reconstructed Human Epidermis test. The accuracy for subcategorizing corrosive substances into categories 1 A (<25 % at 3 min) and 1B/1C (≥25 % at 3 min, <25 % at 60 min) was 83.3 % (25/30). The accuracy of SIT identifying non-irritant (≥50 % at 60 min) was 83.3 % (15/18). FITC-dextran penetration assay showed a similar accuracy, highlighting its value as an alternative endpoint to identify irritants. Collectively, this study demonstrated that the ex vivo porcine skin model may offer a cost-effective, and reliable alternative for skin hazard testing.Copyright © 2025. Published by Elsevier Ltd.
Production of monoclonal antibodies targeting plasma membrane of porcine Y-chromosome-bearing spermChot, Thongkham, Satsook
et alReprod Biol (2025) 25 (2), 101009
Abstract: The pig industry is interested in increasing the number of female piglets by using sexed semen. Immunological sperm sexing is a promising method. This study investigated and produced a monoclonal antibody (MAbs) targeted to plasma membrane epitopes on porcine Y-chromosome-bearing sperm. Two BALB/c mice were immunized with 92.08 % high-purity porcine Y-sperm, which was separated by a cell sorter flow cytometer. The hybridoma cells were a fusion of myeloma cells (P3X63Ag8.653) and splenocyte cells from immunized mice. Indirect ELISA screening for positive antibodies produced by a single clone well (C2B2) with a high titer specific to porcine Y-sperm. The C2B2 clone was used to produce and purify C2B2-MAbs, yielding 2.78 ± 0.78 µg/mL. The C2B2-MAbs was highly specific to Y-sperm (100.00 %) and had a low cross-reactivity with X-sperm (3.25 %). Therefore, the percentage cross-reactivity of C2B2-MAbs was low for conventional sperm from various livestock, including 0.34 % for Angus, 0.38 % for Holstein-Friesian, 0.20 % for goats, and 0.25 % for buffalo. The bright fluorescence of FITC displayed by the C2B2-MAbs bound to the plasma membrane of porcine Y-sperm provided evidence of affinity between them. However, the C2B2-MAbs bound to an X-sperm lacked fluorescence. C2B2-MAbs showed specificity for the plasma membrane of porcine Y-sperm, which can be used in porcine semen sexing in further studies.Copyright © 2025 Society for Biology of Reproduction & the Institute of Animal Reproduction and Food Research of Polish Academy of Sciences in Olsztyn. Published by Elsevier B.V. All rights reserved.
In vivo imaging to trace the dissemination of Aeromonas hydrophila in Common carp (Cyprinus carpio) after intestinal infectionWang, Li, Wang
et alFish Shellfish Immunol (2025)
Abstract: Aeromonas hydrophila (A. hydrophila) is a pathogenic bacterium that often causes serious economic losses to aquaculture and fisheries. Its infection and pathogenesis of fish are currently not fully understood. In this study, A. hydrophila was labeled with Fluorescein Isothiocyanate (FITC) and administered to common carp via gavage to trace its dissemination in common carp (Cyprinus carpio) after intestinal infection by in vivo imaging system. The optimal FITC concentration for labeling was determined to be 30 μg/mL, with a labeling time of 3 h at a bacterial concentration of 108 CFU/mL, with no significant impact on bacterial viability. The fluorescence imaging results of IVIS showed that FITC-Ah entered the internal organs through the carp intestine, first entering the kidneys and hepatopancreas (0.5 h after oral inoculation), and then the brain (around 10 h). Bacterial clearance occured first in the hepatopancreas, followed by the brain, with the slowest clearance in the kidneys. Plate culture, 16S rRNA sequencing, and comparison of clinical symptoms confirmed the reliability of the tracing results. At the molecular level, interleukin-6 (il-6) and interleukin-8 (il-8) showed an upregulation trend, while the expression of interleukin-10 (il-10) initially decreased and then increased in the kidneys, hepatopancreas, and spleen of common carp after oral infection. The results revealed that A. hydrophila can not only infect fish through the intestine, but also mainly invade the hepatopancreas, kidneys, and brain after entering the fish's body via the intestine. Furthermore, the clearance kinetics of A. hydrophila in the internal organs of fish show that it is first cleared from the hepatopancreas, followed by the brain, with the slowest clearance occurring in the kidneys.Copyright © 2025. Published by Elsevier Ltd.
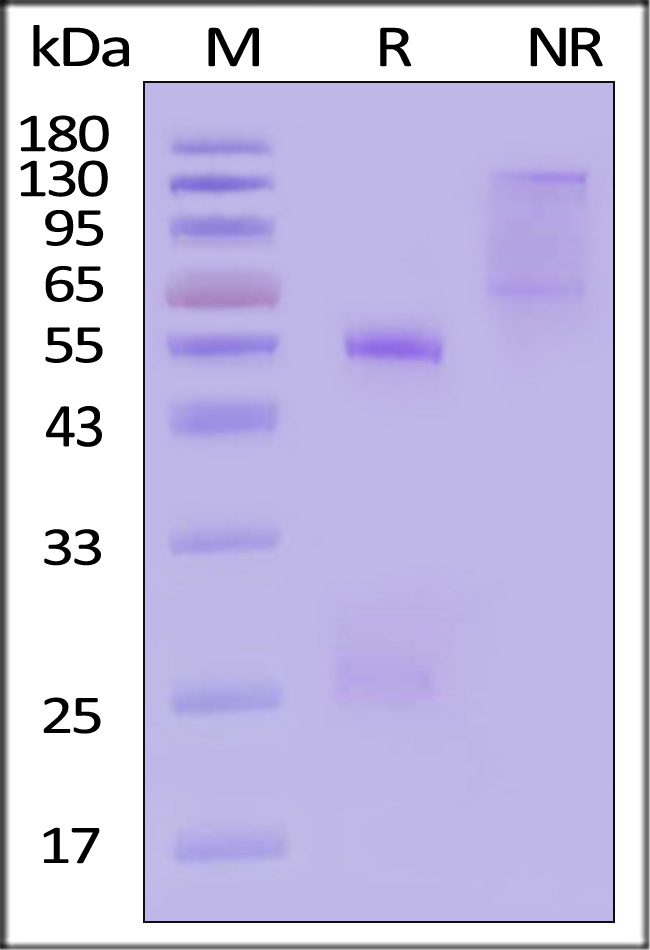
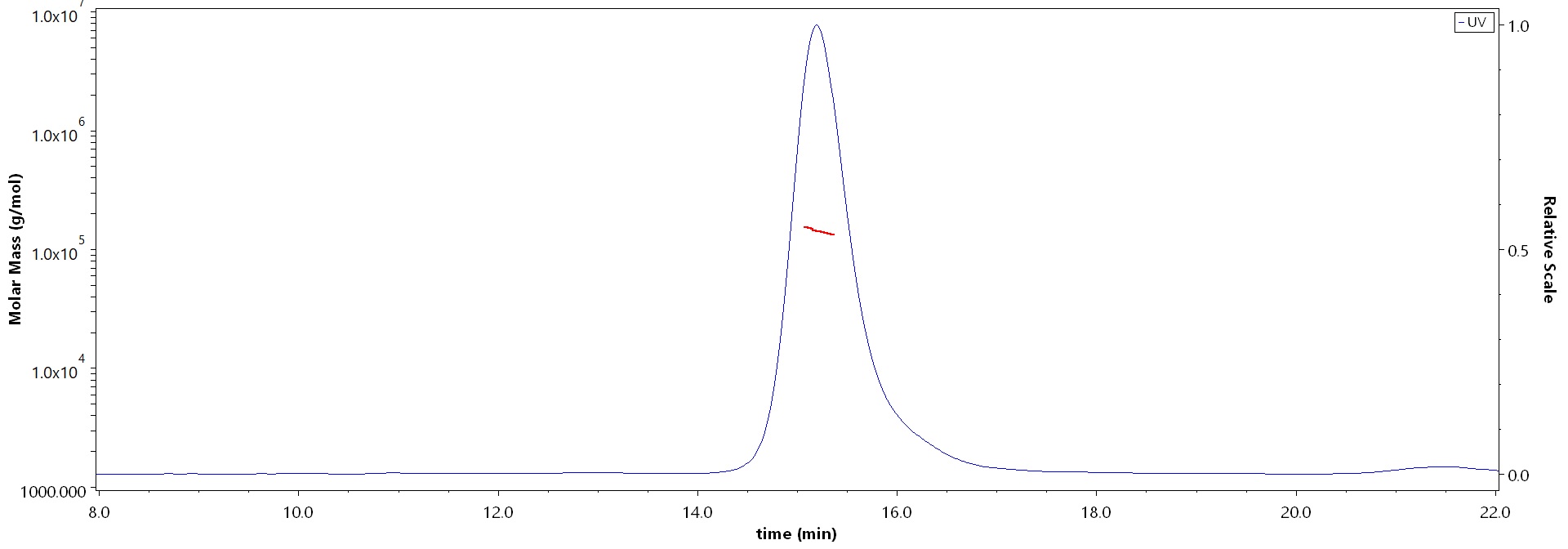























































 膜杰作
膜杰作 Star Staining
Star Staining