- High Recovery - Optimized dissociation process to ensure thorough dissociation between drug and Protein A leached, to get satisfactory recovery even for samples of low recovery.
- Universality - Suitable for detection of natural or structurally conserved recombinant forms of Protein A and alkaline-resistant Protein A variants, such as MabSelect SuRe™ , MaXtar® ARPA ligand (Bio-Link Co.), UniMab® 50 Protein A (NanoMicro), MabSelect SuRe™ and other ligands.
- Fast time to results - less than 2 hours.
- Accuracy - Tracebility of Protein A standards against BSA Standard with validated pharmacopoeia quantitation methodology.
- Efficient sample processing methods - adding denaturing reagents to prepare samples that the sample processing is just about 15 mins.
- High sensitivity - Sensitivity < 40 pg/mL of recombinant forms of Protein A and alkaline-resistant Protein A variants.
- High IgG tolerance - Accurately quantify protein A in up to 20 mg/mL antibody.
- Excellent buffer compatibility - Compatible with various buffer systems.
- Extensive validation - Comprehensive verification of specificity, sensitivity, precision, accuracy, applicability, and other aspects.
产品描述(Product Details)
| Assay Type | Sandwich-ELISA |
| Analyte | Protein A |
| Format | 96T(8×12 strips) |
| Regulatory Status | RUO |
| Sensitivity | <40pg/mL |
| Standard Curve Range | 50 pg/mL-1600 pg/mL(Protein A) 50 pg/mL-3200 pg/mL(PrismA) |
| Assay Time | 2 hr |
| Suitable Sample Type | For the quantitative determination of recombinant Protein A, alkaline-resistant Protein A, PrismA and MaXtar® ARPA ligand Protein A. |
| Sample volume | 25 μL |
背景(Background)
Protein A is a cell wall protein of Staphylococcus aureus, it has a variety of specific biological characteristics. Due to its high affinity with the Fc part of certain immunoglobulins (especially IgG), it is widely used in the purification of biopharmaceuticals (such as antibodies, vaccines, etc.). However, during the purification, protein A may leach from the purification column and result in contamination of the antibody drugs prepared. Once the remaining protein A enters the human body, it will easily activate the immune response of the organism, and there is a safety risk, so there are strict regulations on the residual level of Protein A in antibody drug preparations. Therefore, the detection of residual Protein A in antibody drugs purified from Protein A purification column is a key quality control step in the production process of antibody drug preparations.
The resDetect™ Universal Protein A Quick ELISA Kit (Boiling-free) can detect protein A or unnatural protein A variants within 2 hours, it is high sensitive and easy to operate because the sample processing has been changed from the original boiling method to adding denaturing reagents. The sample processing process has been shortened by one hour. Whether in upstream small-scale trials or downstream large-scale of antibody production processes, this kit can help you to accurate analysis of samples, monitor the protein A levels and ensure product quality.
应用说明(Application)
The kit is developed for the detection of natural or structurally conserved recombinant forms of Protein A and a recombinant form of Protein A with very significant structural differences from natural Protein A such as MabSelect SuReTM in Bioprocess manufacturing applications. It is used as a tool to aid in optimal purification process development and in routine quality control of in-process streams as well as final product.
It is for research use only.
存储(Storage)
Unopened kit should be stored at 2°C-8°C upon receiving.
Find the expiration date on the outside packaging and do not use reagents past their expiration date.
The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
组分(Materials Provided)
| ID | Components | Size |
| RES029-C01 | Pre-Coated Anti-Protein A Antibody Microplate | 1 plate(8×12 strips) |
| RES029-C02A | Alkali-Tolerant Recombinant Protein A Standard (1μg/mL) | 100 μL |
| RES029-C02B | MaXtar® ARPA ligand Protein A Standard (Bio-Link Co.) (1μg/mL) | 100 μL |
| RES029-C02C | Recombinant PrismA Standard (1μg/mL) | 100 μL |
| RES029-C03 | Recombinant Protein A Standard (1μg/mL) | 100 μL |
| RES029-C04 | Biotin-Anti-Protein A Antibody | 1.5 mL |
| RES029-C05 | Streptavidin-HRP | 10 μg |
| RES029-C06 | 10×Sample Dilution Buffer | 15 mL |
| RES029-C07 | Denaturation Buffer | 15 mL |
| RES029-C08 | 20×Washing Buffer | 30 mL |
| RES029-C09 | Antibody Dilution Buffer | 15 mL |
| RES029-C10 | Streptavidin-HRP Dilution Buffer | 15 mL |
| RES029-C11 | Substrate Solution | 12 mL |
| RES029-C12 | Stop Solution | 8 mL |
原理(Assay Principles)
The resDetect™ Universal Protein A Quick ELISA Kit (Boiling-free) is used to measure the levels of protein A and protein A variants by employing a standard sandwich-ELISA format. The micro-plate in the kit has been pre-coated with anti-protein A polyclonal antibody. Firstly, the standard samples provided in kit and your samples are treated with Denaturation Buffer to dissociation of protein A and antibody, stand a few minute. Before adding standards and samples, add the Biotin-Anti-Protein A Antibody to the plate to ensure that the standard samples are neutralized by the Biotin-Anti-Protein A Antibody buffer solution and protect the pre-coated antibody on the plate. Then, add the standard samples and your samples to the plate and form Antibody-antigen (Protein A) - biotinylated antibody complex, incubate and wash the wells. Next add Horseradish peroxidase conjugated streptavidin (Streptavidin-HRP) to the plate, incubate and wash the wells to remove any unbound reactants. At last, load the tetramethylbenzidine (TMB) substrate into the wells and monitor a blue color. The reaction is stopped by the addition of a stop solution and the color turns yellow. The intensity of the absorbance can be measured at 450nm and 630nm on a microtiter plate reader. The OD Value reflects the amount of protein A.
典型数据-Typical Data Please refer to DS document for the assay protocol.

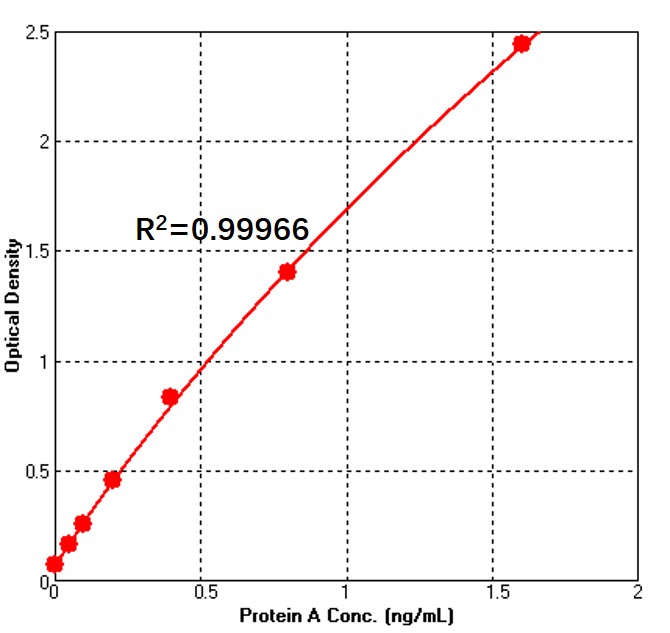
Detection of Recombinant Protein A by sandwich-ELISA Assay.
Immobilized Anti-Protein A Antibody can bind Recombinant Protein A. Detection was performed using Biotin-Anti-Protein A Antibody with sensitivity of 50 pg/mL (QC tested). For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
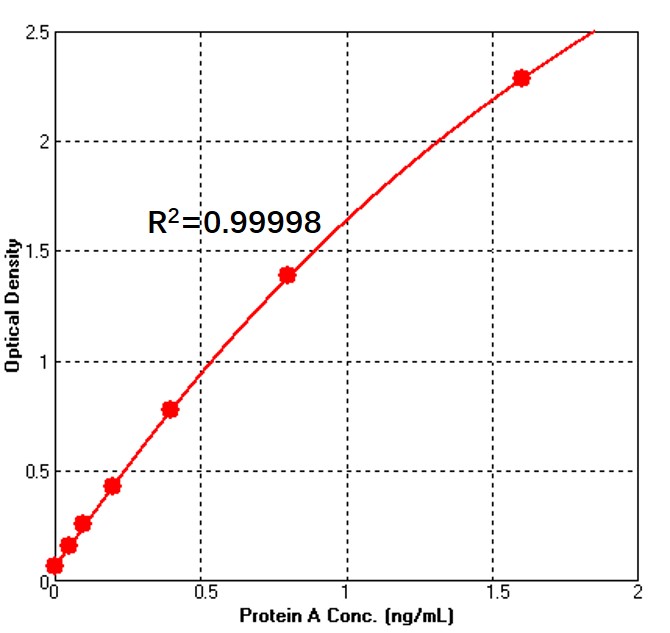
Detection of Alkali-Tolerant Recombinant Protein A by sandwich-ELISA Assay.
Immobilized Anti-Protein A Antibody can bind Alkali-Tolerant Recombinant Protein A. Detection was performed using Biotin-Anti-Protein A Antibody with sensitivity of 50 pg/mL (QC tested). For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
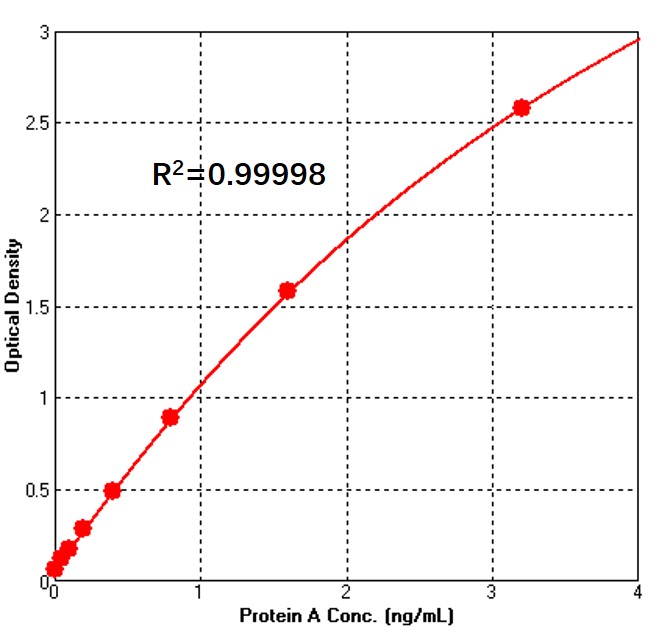
Detection of MaXtar® ARPA ligand Protein A (Bio-Link Co.) by sandwich-ELISA Assay.
Immobilized Anti-Protein A Antibody can bind MaXtar® ARPA ligand Protein A (Bio-Link Co.). Detection was performed using Biotin-Anti-Protein A Antibody with sensitivity of 50 pg/mL (QC tested). For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
Detection of Recombinant PrismA by sandwich-ELISA Assay.
Immobilized Anti-Protein A Antibody can bind Recombinant PrismA. Detection was performed using Biotin-Anti-Protein A Antibody with sensitivity of 50 pg/mL (QC tested). For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
验证(Validation)
批内差异(Intra-Assay Statistics)
Four samples of known concentration were tested ten times on one plate to assess intra-assay precision.
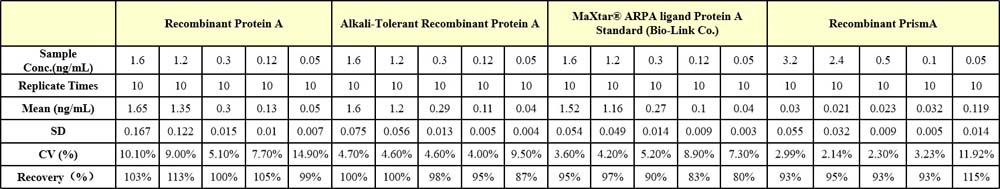
批间差异(Inter-Assay Statistics)
Four samples of known concentration were tested in three separate assays to assess inter-assay precision.
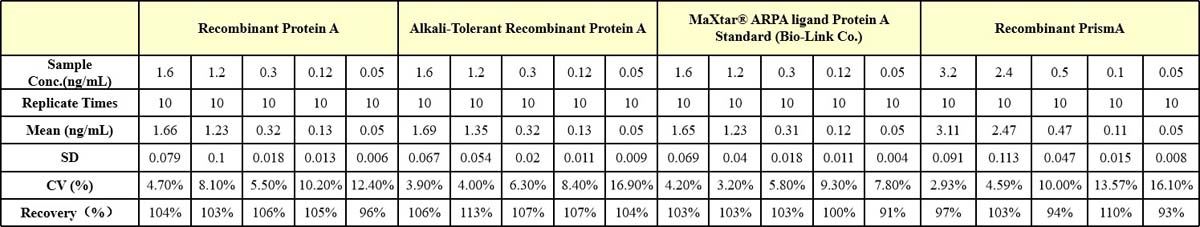
回收率(Recovery)
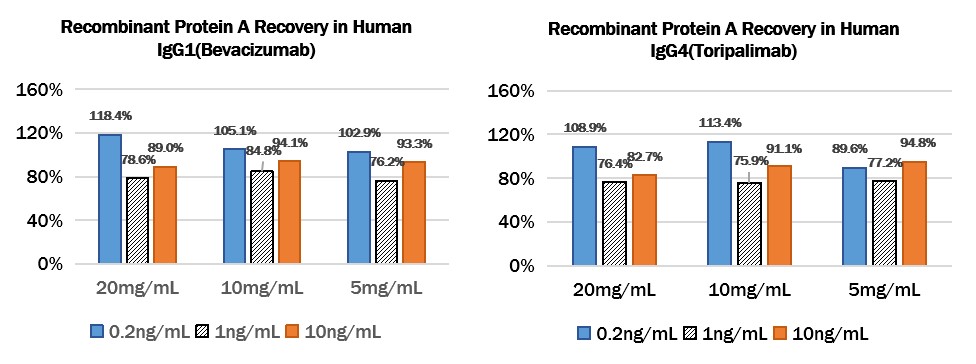
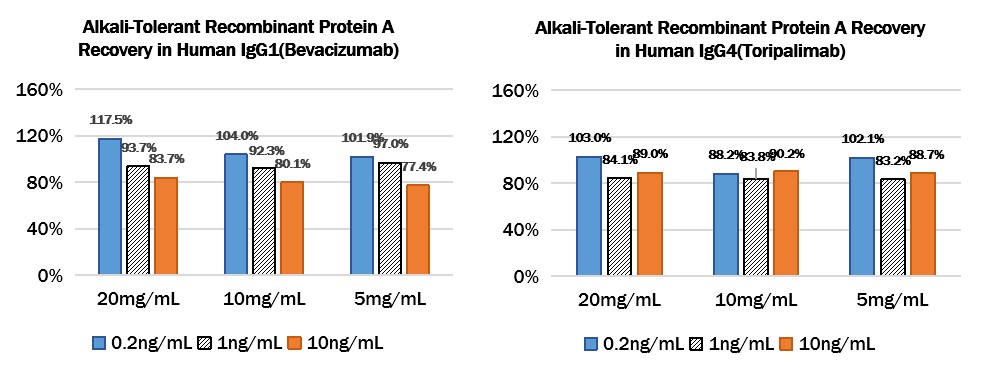
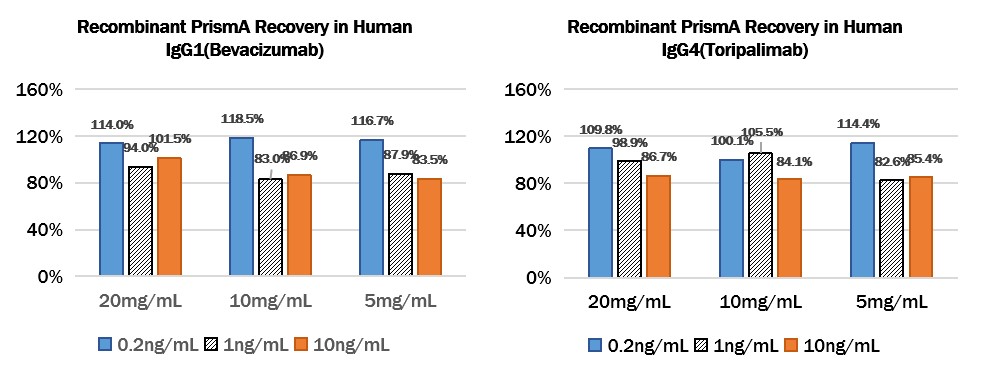
Add different concentrations of Protein A (0.2ng/mL、1ng/mL、10ng/mL) to different concentrations of Human IgG1 (Bevacizumab) (20mg/mL、10mg/mL、5mg/mL) or Human IgG4 (Toripalimab) (20mg/mL、10mg/mL、5mg/mL), then dilute the antibodies to a reasonable range, then test and calculated the concentration of protein A to give the recovery rate.
Add Recombinant Protein A to Human IgG1 (Bevacizumab) or Human IgG4 (Toripalimab):
Add Alkali-Tolerant Recombinant Protein A to Human IgG1 (Bevacizumab) or Human IgG4 (Toripalimab):
Add MaXtar® ARPA ligand Protein A (Bio-Link Co.) to Human IgG1 (Bevacizumab) or Human IgG4 (Toripalimab):
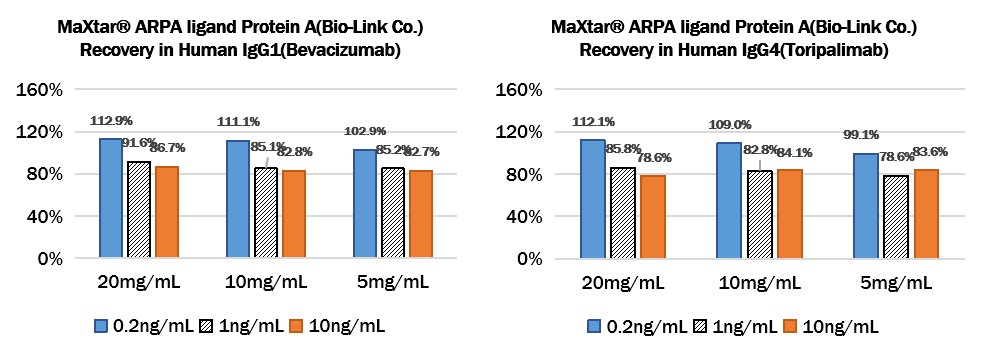
Add Recombinant PrismA to Human IgG1 (Bevacizumab) or Human IgG4 (Toripalimab):
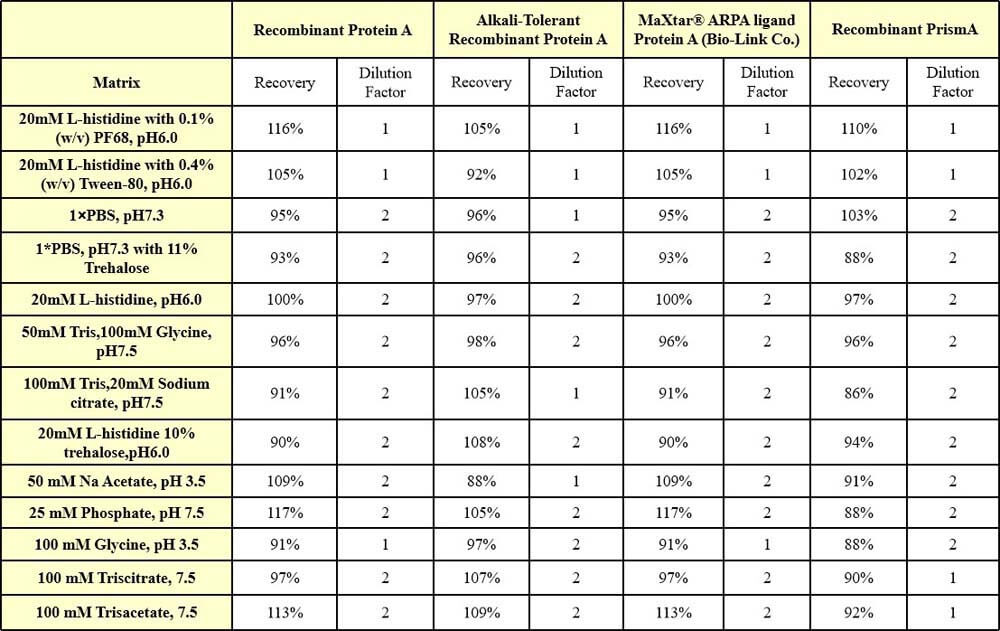
干扰效应(Interference effect)
We have conducted interference effect test about frequently-used buffers, they have excellent buffer compatibility. For specific buffers, it is recommended that you verify recovery to determine the minimum dilution ratio.
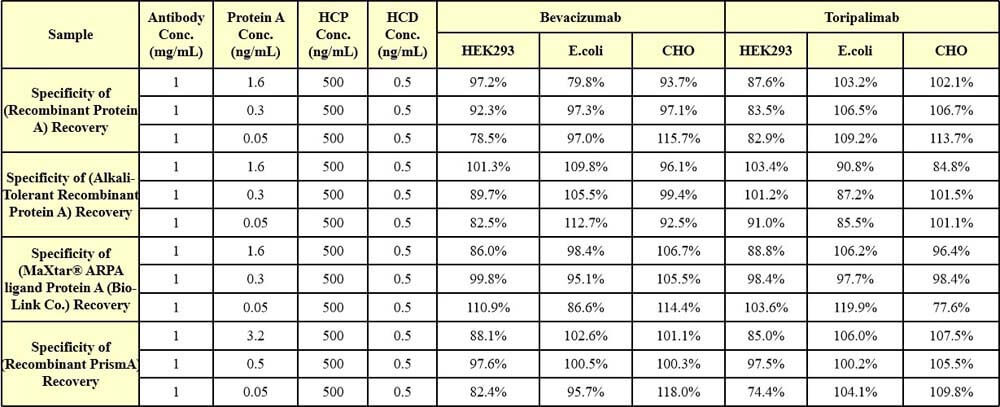
专属性(Specificity)
Host cell protein (HCP 500 ng/mL) and host cell DNA (HCD 0.5 ng/mL) of HEK293, E.coli or CHO systems were added to human IgG1 (Bevacizumab, 1mg/mL) and human IgG4 (Toripalimab, 1mg/mL), respectively, which were higher than the usual quality standard limit. Then high, medium, and low concentrations of Protein A were added, respectively, and the ratio of Protein A recovery in the Protein A added samples without HCP and HCD was added as the specificity verification index. verification index.























































 膜杰作
膜杰作 Star Staining
Star Staining