Fabrication of Bioconjugates Clacked Chitosan-Coated SN-38 Liposomal Nanoparticles: Improved Precise Chemotherapy Efficacy in Lung Cancer Cells and Its ApoptosisZhang, Sun, Liu
Biotechnol Appl Biochem (2025)
Abstract: Employing an active targeting method with monoclonal antibodies for chemotherapeutics-loaded nanocarriers represents a promising option to enhance the specific drug delivery and alleviate the detrimental effects of chemotherapeutic agents. Targeted delivery to the human epidermal growth factor receptor-2 (HER2), which is overexpressed in HER2+ lung cancerous cells, can be accomplished by conjugating nanoparticles with a monoclonal antibody (anti-HER2). We developed trastuzumab (TZ)-conjugated chitosan iodoacetamide (CsIA)-coated liposomal nanoparticles carrying SN-38 (TZ-SN-CsIA LNPs) as a lung-targeted delivery. CsIA was used to develop trastuzumab-clacked nanoparticles (TZ LNPs). The structure, physicochemical characteristics, SN-38 encapsulation, SN-38 release, and anticancer properties of the LNPs were established. The TZ LNPs were spherical, measuring around 77 nm in diameter, and exhibited a positive zeta potential; upon drug incorporation, the diameter of the TZ LNPs enlarged. A sustained, 24-h SN-38 release from the nanocarriers was accomplished. TZ LNPs demonstrated substantial cellular accumulation and markedly enhanced anticancer efficacy against HER2+ Calu-3 lung adenocarcinoma cancer cells compared to the drug solution and unconjugated LNPs. Consequently, TZ-SN-CsIA LNPs may be a potential nanocarrier for HER2+ lung cancer.© 2025 International Union of Biochemistry and Molecular Biology, Inc.
Impact of DNA repair deficiency on sensitivity to antibody-drug conjugate (ADC) payloads in bladder cancerP Shanmugam, Zhou, Stelter
et alBladder Cancer (2025) 11 (1), 23523735251317865
Abstract: Enfortumab vedotin (EV) and Sacituzumab govitecan (SG) are antibody-drug conjugates (ADCs) with demonstrated activity in advanced bladder cancer. A subset of bladder tumors harbors a DNA repair deficiency in either the homologous recombination (HR) or nucleotide excision repair (NER) pathway that has the potential to impact sensitivity to specific classes of therapeutics.Define the impact of HR or NER deficiency on sensitivity to ADC payloads alone or in combination with DNA repair targeted agents in bladder cancer.Isogenic cell pairs with versus without HR or NER deficiency were profiled using DNA repair and drug sensitivity assays. Sensitivity to the ADC payloads monomethyl auristatin E (MMAE) and SN-38 alone or in combination with small molecule inhibitors of poly(ADP-ribose) polymerase (PARP), ATR, or USP1 were measured using cell viability assays.BRCA2 loss was sufficient to confer an HR deficient phenotype and increase sensitivity to cisplatin and PARP inhibition in bladder cancer cell lines. HR deficiency, but not NER deficiency, increased sensitivity to MMAE and SN-38 in bladder cancer cells. The combination of SN-38 and PARP inhibition displayed synergistic cell killing independent of HR or NER status.HR and NER deficiency have distinct impacts on sensitivity to cisplatin and ADC payloads in bladder cancer preclinical models.© The Author(s) 2025.
Calcium-dependent adhesion protein CDH18, a potential biomarker for prognosis in uterine corpus endometrial carcinomaTang, Dang, Qiu
et alFront Mol Biosci (2025) 12, 1530253
Abstract: Uterine corpus endometrial carcinoma (UCEC) is one of the most common cancers in women, yet lacks specific and sensitive tumor markers for diagnosis, as traditional markers like CA125 show limited specificity. This study investigates the clinical significance and prognostic value of CDH18, a calcium-dependent adhesion protein linked to tumor progression, in UCEC.Clinical data from UCEC patients were sourced from The Cancer Genome Atlas (TCGA) database. Pan-cancer analysis, differential expression examination, and survival analysis were conducted to investigate the differential expression of the calcium associated protein-CDH18 and its prognostic relevance. CDH18 mutations in UCEC were examined using the cBioPortal database. Additional analyses included functional enrichment, tumor mutational burden, tumor microenvironment (TME) estimates via ESTIMATE, and immune infiltration assessment to clarify CDH18's potential mechanisms in UCEC. Drug sensitivity testing was utilized to identify more suitable therapeutic options for patients. Immunofluorescence staining (IF) and Real-Time Polymerase Chain Reaction techniques (RT-PCR) confirmed CDH18 expression in UCEC tumor.CDH18 expression was markedly increased in UCEC and showed a significant association with poorer prognosis, which was confirmed by our IF and RT-PCR results. Thirteen mutation sites were identified, and survival analysis showed that patients with higher CDH18 expression had shorter overall survival. The expression of CDH18 was confirmed to be an independent predictor of overall survival by multivariate COX regression analysis. Additionally, a predictive nomogram model was developed to accurately forecast outcomes for individuals with UCEC. Correlation analysis revealed that CDH18 expression exhibited a negative correlation with CD8 T cell levels and a positive correlation with resting NK cell and macrophage M2 levels. In the group with high CDH18 expression, the IC50 values for (5Z)-7-Oxozeaenol, AG-014699, CEP-701, Mitomycin C, PD-0325901, PD-0332991, PHA-665752, SL 0101-1, and SN-38 were notably elevated.CDH18 is a novel promising biomarker in UCEC, uniquely associating tumor progression, immune modulation, and chemotherapy resistance, offering enhanced prognostic accuracy and guiding individualized therapeutic strategies for improved patient outcomes.Copyright © 2025 Tang, Dang, Qiu, Zhou, Ling, Zhang, Peng, Li, Liu, Liao, Mei, Xie, Sun, Huang, Du and Song.
Understanding the Toxicity Profile of Approved ADCsBallestín, López de Sá, Díaz-Tejeiro
et alPharmaceutics (2025) 17 (2)
Abstract: Background: Antibody-drug conjugates (ADCs) represent a novel therapeutic class that combines an antibody against a tumor-associated antigen (TAA), a payload, and a linker that binds these two components. Serious adverse events (SAEs), particularly those of grade 3 (G3) or higher, frequently contribute to the abandonment of ADCs during clinical development. Methods: In this study, we analyzed the toxicity profiles of all approved ADCs, aiming to uncover correlations between their safety profiles and the specific characteristics of their components. Results: In our analysis, dose reductions, dose delays, treatment discontinuations, and ≥G3 toxicities were not significantly different across payload types. Similarly, no association was found between the payload mechanism of action and ≥G3 toxicities, including anemia, neutropenia, febrile neutropenia, thrombocytopenia, and diarrhea. By exploring the specific toxicities of ADCs observed by organ, we identified that most were related to the payload mechanism of action, like the ≥G3 diarrhea observed in 10% of patients treated with sacituzumab govitecan (the payload SN-38 is the active metabolite of irinotecan), and very few were related to the presence of the TAA in normal tissue (presence of Nectin-4 in skin and ≥G3 rash toxicity in 14% of patients treated with enfortumab vedotin). In line with this, no major differences in ≥G3 toxicities were identified in studies with different levels of the TAA (trastuzumab deruxtecan in Destiny Breast Studies with different HER2 expression levels). Conclusions: Our analysis reveals that most ADC toxicities are driven by the payload's effects on non-transformed tissues; however, a detailed analysis of each ADC component should be taken into consideration.
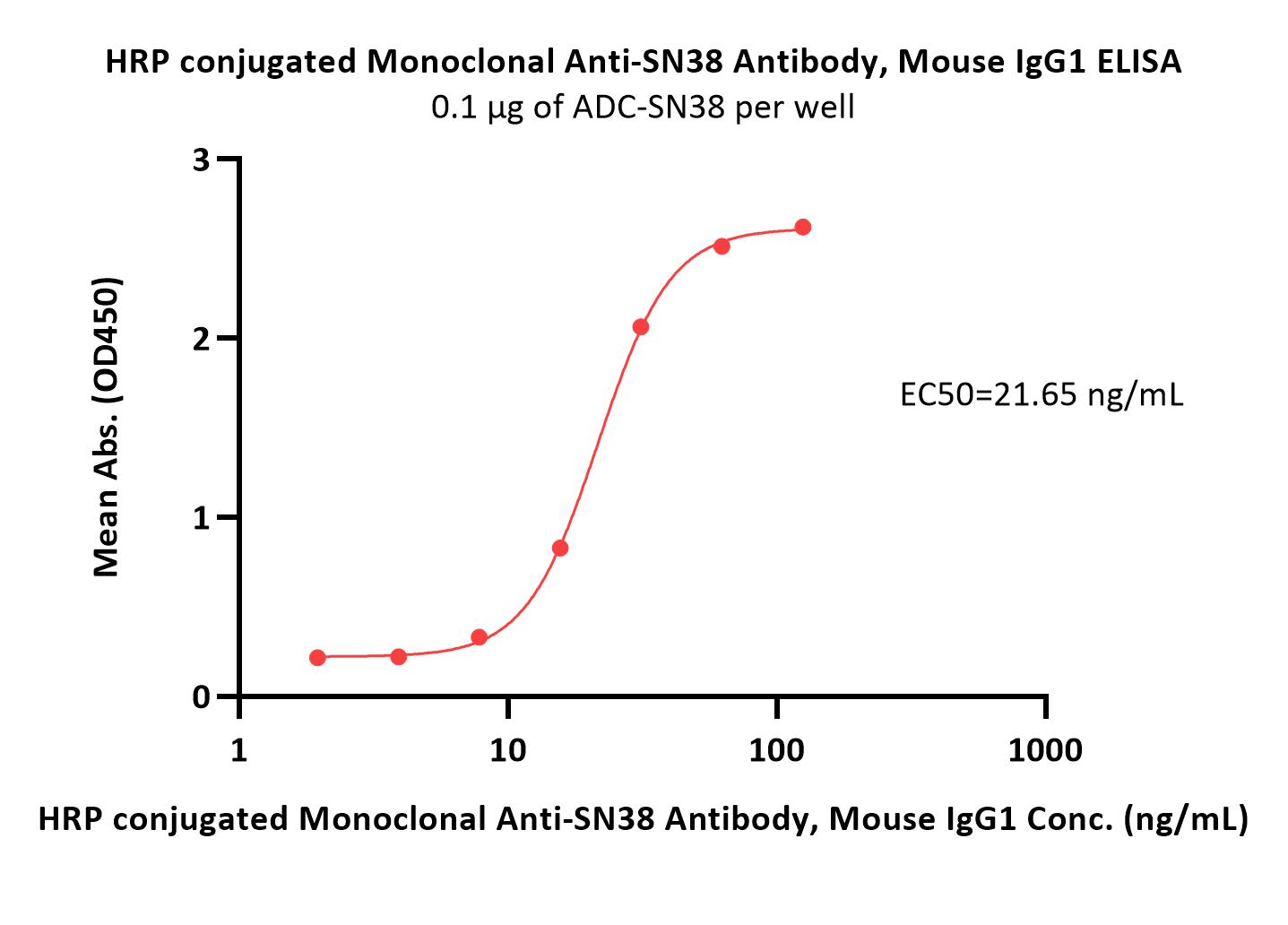























































 膜杰作
膜杰作 Star Staining
Star Staining