产品描述(Product Details)
To expedite in-process and lot-release testing, the Mycoplasma rapid detection kit, based on Nucleic Acid Amplification Technology (NAT), has been developed and validated to meet the requirements outlined in European Pharmacopeia Chapter 2.6. 7.
Combined with ACRO's mycoplasma DNA Sample Preparation kit (OPA-E101), this kit sensitively and reliably detects mycoplasma contamination in biological products, meeting or exceeding the regulatory guidance of 10 CFU/mL. The assay eliminates the need for time-consuming culture-based methods, simplifying the mycoplasma testing process and yielding results in 2-2.5 hours with high efficiency.
产品特性(Features)
- • Broad Coverage: Covers over 250 Mollicutes (Mycoplasma, Acholeplasma and Spiroplasma) species, including all mycoplasma species listed in Pharmacopoeias (EP, USP, JP)
- • Strong Specificity: Developed with multiple primers and probes targeting mycoplasma species' 16s rRNA, with no cross-reactivity with closely related non-mycoplasma strains
- • High Sensitivity: Fully compliant with or superior to regulatory guidance of 10 CFU/mL
- • Convenient Operation: Designed for single-well testing, with the kit containing essential components for easy use
- • Comparable Results: Detection results from the kit are comparable to those from culture-based methods
- • High-quality: This Kit is manufactured in GMP-like facility and alignment with the ISO 13485 standard
应用说明(Application)
The kit is used for detection of mycoplasma contamination in cells and bioproduct media.
For use in quality control/manufacturing process only.
It is for research use only.
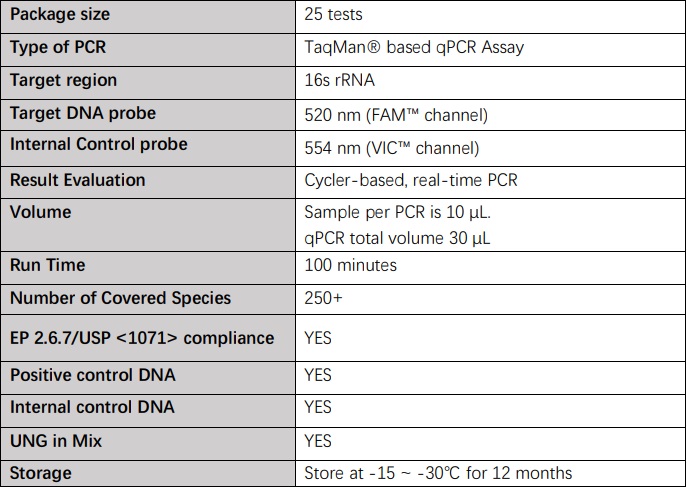
技术参数(Technical Specifications)
使用提示(Attention)
If your experimental process requires sample preparation, please purchase and conduct the experiment using the sample preparation kits recommended in the table to ensure that the buffers used in both the sample preparation and resDNA detection are consistent.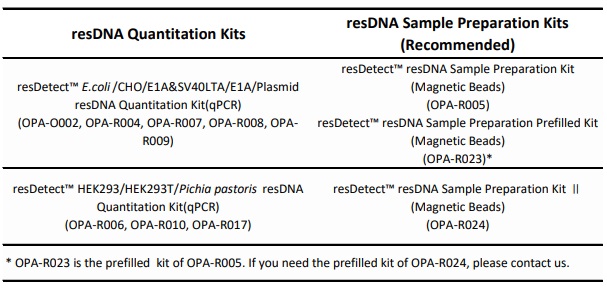
组分(Materials Provided)
| ID | Components | Size |
| OPA-S101-01 | PC Powder | 1 tube |
| OPA-S101-02 | Purple-capped empty tube | 1 tube |
| OPA-S101-03 | DNA Dilution Buffer | 1.5 mL×2 |
| OPA-S101-04 | Internal Control DNA | 300 μL |
| OPA-S101-05 | 2×qPCR Master Mix | 400 μL |
| OPA-S101-06 | Myco Primer&Probe Mix | 100 μL |
| OPA-S101-07 | Dnase/Rnase-Free Water | 1.0 mL |
背景(Background)
During the production of biological products (cell isolation, modification, and proliferation), contamination by mycoplasma poses potential risks to patients. Therefore, it is crucial to ensure that the final products are free from mycoplasma contamination during release testing. Traditionally, assessing whether biological products have been contaminated relied on conventional microbiological culture procedures using liquid media and agar media (culture method or indicator cell culture method). However, with the advancement of Biopharmaceuticals and Advanced Therapy Medicinal Products (ATMPs), culture-based methods no longer meet the rapid release requirements.























































 膜杰作
膜杰作 Star Staining
Star Staining