产品描述(Product Details)
| Assay Type | Competitive-ELISA |
| Analyte | Gentamicin |
| Format | 96T(8×12 strips) |
| Regulatory Status | RUO |
| Sensitivity | 0.1ng/mL |
| Standard Curve Range | 0.1 ng/mL-3.2 ng/mL |
| Assay Time | 1hr 20 min |
| Suitable Sample Type | For the detection and quantitative determination of Gentamicin residues in plasmid DNA raw materials for cell and gene therap. |
| Sample volume | 50ul |
背景(Background)
Residues of gentamicin are prone to occur in the production process of biological products, which can easily lead to abnormal reactions in the human body. Therefore, the residual amount of gentamicin in biological products or semi-finished products of biological products should be strictly controlled.
应用说明(Application)
The kit is developed for the detection of Gentamicin in drug products or semi-manufactures.
It is for research use only.
存储(Storage)
1. Unopened kit should be stored at 2℃-8℃ upon receiving.
2. Find the expiration date on the outside packaging and do not use reagents past their expiration date.
3. The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
组分(Materials Provided)
| ID | Components | Size |
| RES-A079-C01 | Gentamicin Coated Plate | 1 plate(8×12 strips) |
| RES-A079-C02 | Gentamicin Standard | 0.1152ug |
| RES-A079-C03 | HRP-Anti-Gentamicin Antibody | 6mL |
| RES-A079-C04 | 1×Dilution Buffer | 50mL |
| RES-A079-C05 | 20xWashing Buffer | 50mL |
| RES-A079-C06 | Substrate Solution | 12mL |
| RES-A079-C07 | Stop Solution | 7mL |
原理(Assay Principles)
The Gentamicin quantitative detection kit adopts the indirect competitive ELISA method, and the pre-coated conjugated Gentamicin antigen on the microstrip competes with the residual Gentamicin in the sample to bind the enzyme-labeled anti-Gentamicin monoclonal antibody, and then uses a microplate reader to detect the absorbance value by adding TMB substrate, and the absorbance value is negatively correlated with the content of kanamycin in the sample. The kit takes only about one hour and 20 minutes to operate and has a linear range of 0.1 ng/mL to 3.2 ng/mL.
典型数据-Typical Data Please refer to DS document for the assay protocol.
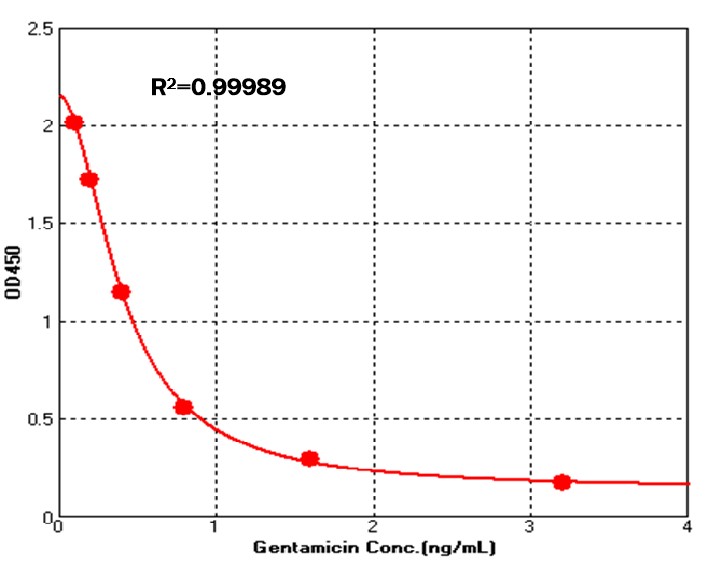
Detection of Gentamicin by competitive ELISA Assay
Immobilized Gentamicin antigen competes with the residual Gentamicin in the sample to bind the enzyme-labeled anti-Gentamicin monoclonal (QC tested). For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
验证(Validation)
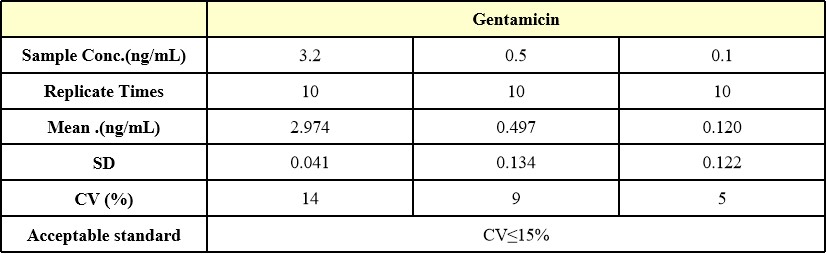
批内差异(Intra-Assay Statistics)
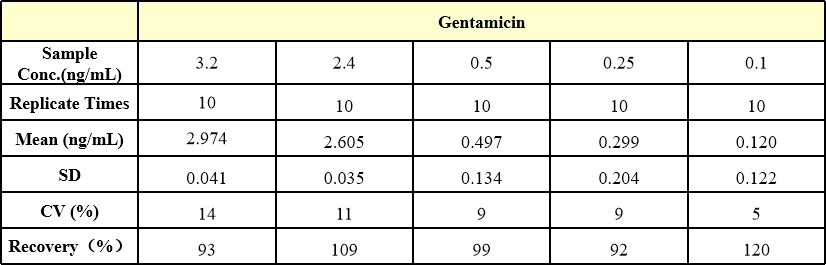
Three samples of known concentration were tested ten times on one plate to assess intra-assay precision , Intra-Assay Precision CV≤15%.
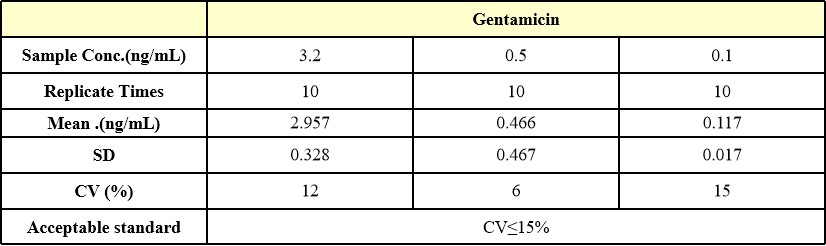
批间差异(Inter-Assay Statistics)
Three samples of known concentration were tested in ten separate assays to assess inter-assay precision, Inter-Assay Precision CV≤15%.
准确度(Accuracy)
Three samples of different concentration were tested ten times to assess Accuracy , Accuracy recovery rate 75-120%.
干扰效应(Interference effect)
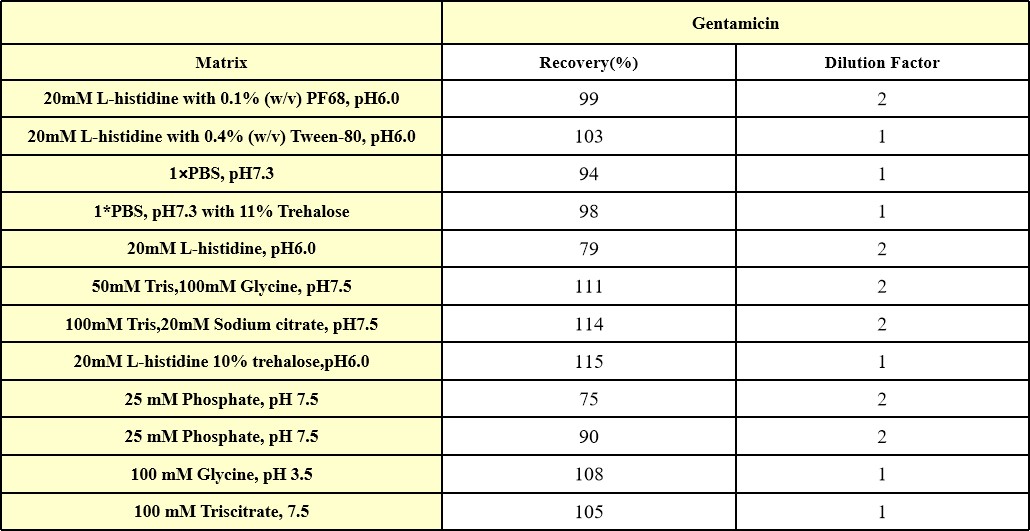
We have conducted interference effect test about frequently-used buffers, they have excellent buffer compatibility. For specific buffers, it is recommended that you verify recovery to determine the minimum dilution ratio.
专属性(Specificity)
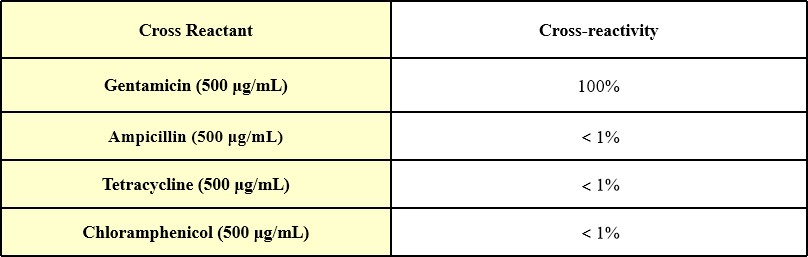
When 500 μg/mL ampicillin, tetracycline and chloramphenicol were added into the sample diluent, no cross-reactivity was observed
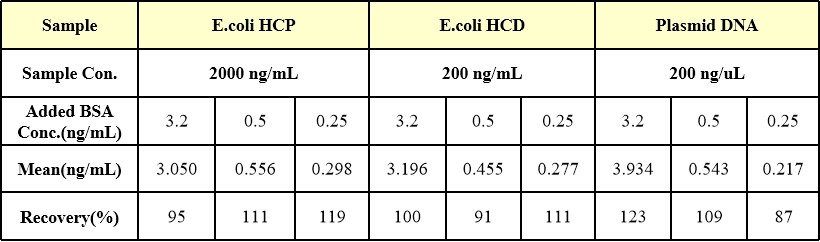
High, medium and low concentrations of BSA were added to MDCK( 2×106 cells/mL), HEK293( 3.5×106 cells/mL) , CHO( 2×106 cells/mL) and T-lymphocyte( 2.15×106 cells/mL) the recovery rate of BSA was used as the specific validation index.























































 膜杰作
膜杰作 Star Staining
Star Staining