| Product | Size | Amount |
| ActiveMax® Human BCMA μBeads, premium grade (for cells) | 2.5 mg | 2.5 × 10⁷ beads |
| ActiveMax® Human BCMA μBeads, premium grade (for cells) | 10 mg (2.5 mg × 4) | 1.0 × 10⁸ beads |
背景(Background)
ActiveMax® Human BCMA μBeads, premium grade (for cells) are produced under sterile manufacturing conditions (ISO 5), and no animal- or human-derived components are used throughout the production process. It is produced under our rigorous quality control system that includes a comprehensive set of tests including sterility and endotoxin tests.
表达区间及表达系统(Source)
ActiveMax® Human BCMA μBeads, premium grade (for cells) are uniform, superparamagnetic beads of 5.5 µm with streptavidin on its surface and coupled with biotinylated Human BCMA Protein, expressed from human 293 cells (HEK293) and contains AA Met 1 - Ala 54 (Accession # Q02223-1).
应用说明(Application)
ActiveMax® Human BCMA μBeads, premium grade (for cells) are designed to stimulate in vitro BCMA-specific CAR-T cells or UCAR-T cells, similar to the tumor cell lines that express human BCMA antigen. It can be used as follows:
Evaluating the characteristics of CAR-T cells or UCAR-T cells.
In vitro expansion of BCMA-specific CAR-T cells or UCAR-T cells.
In vitro enrichment of BCMA-specific CAR-T cells or UCAR-T cells.
重构方法(Reconstitution)
See Certificate of Analysis (CoA) for detailed instruction.
存储(Storage)
This product is stable in storage under the following conditions: -20˚C for 12 months in lyophilized state. -70°C for 3 months under sterile conditions after reconstitution.
Please avoid repeated freeze-thaw cycles after reconstitution. Immediate use after reconstitution is highly recommended.
无菌(Sterility)
Negative
内毒素(Endotoxin)
Less than 0.002 EU per μg by the LAL method.
注意事项(Important Note)
This product is for research use only and not intended for therapeutic or in vivo diagnostic use.
制剂(Formulation)
Please contact us for detailed information.
Contact us for customized product form or formulation.
质量管理控制体系(QMS)
典型数据-Typical Data Please refer to DS document for the assay protocol.
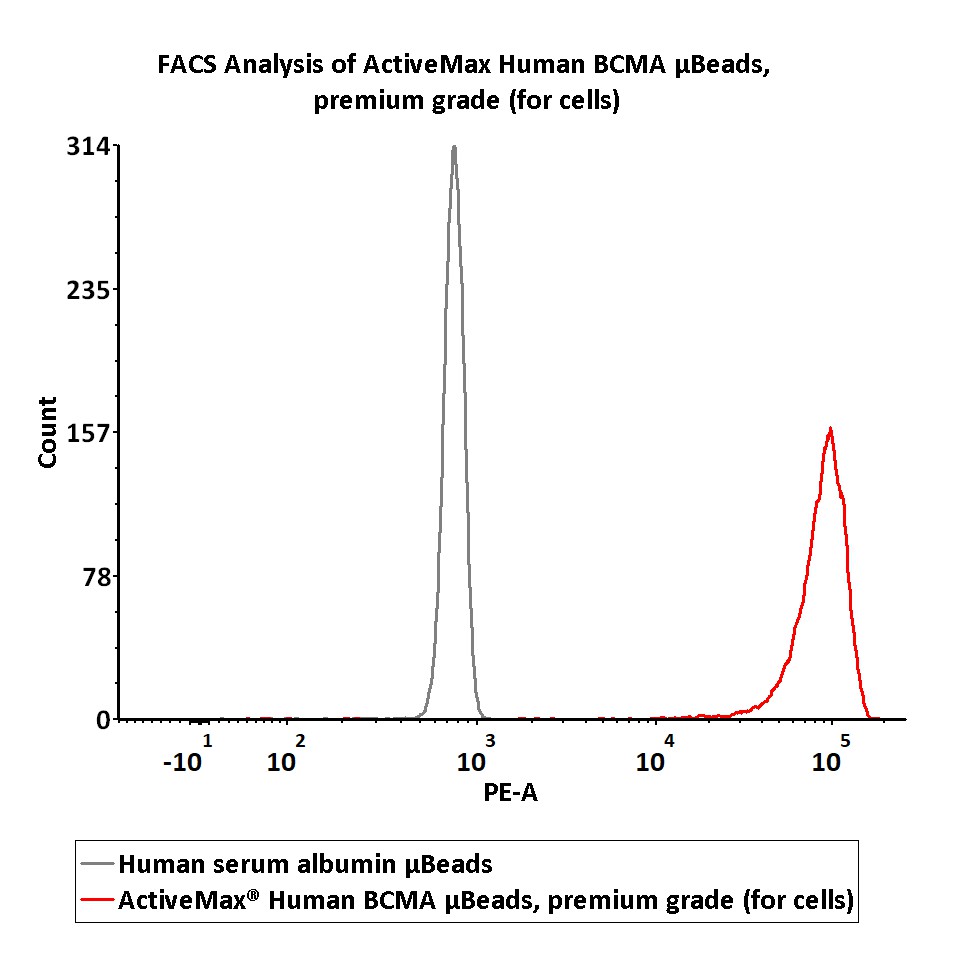
Assay of human BCMA protein on the μBeads surface by Flow cytomtry. The human BCMA conjugated on the μBeads (Cat. No. MBS-C004) surface were fluorescently stained using PE labeled anti-human BCMA antibody and analyzed by flow cytometry.























































 膜杰作
膜杰作 Star Staining
Star Staining






 & Cat.No.
& Cat.No.