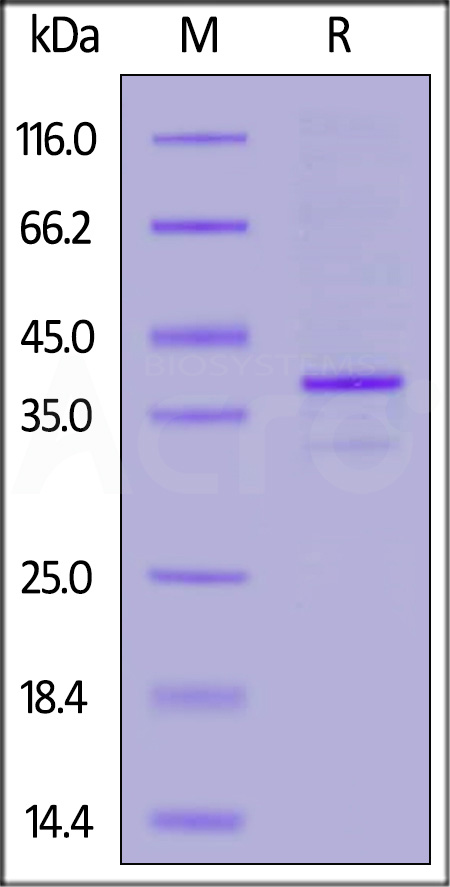
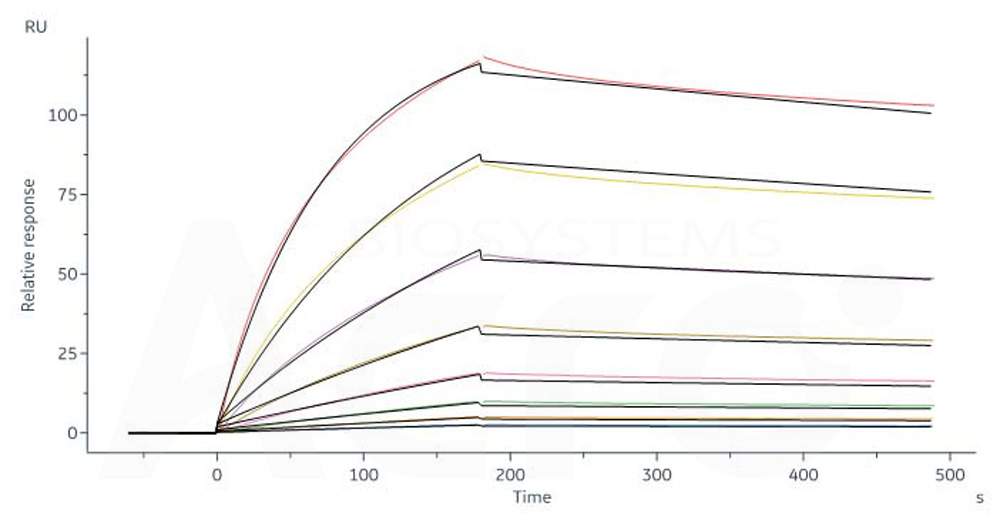
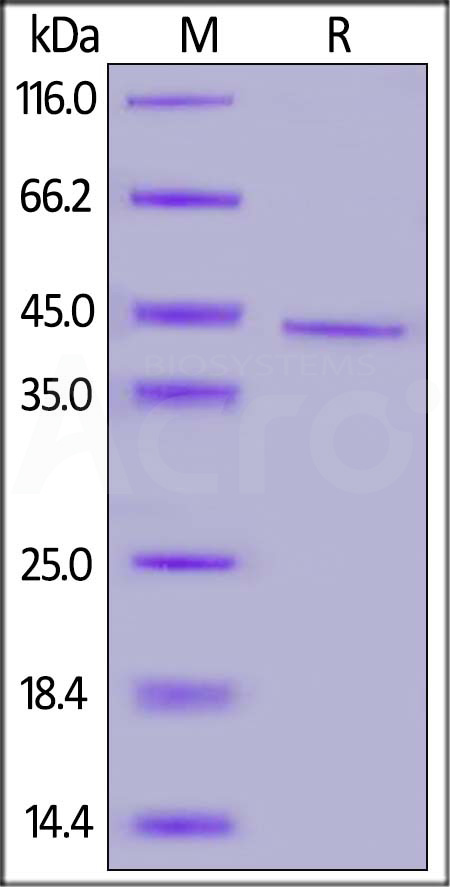
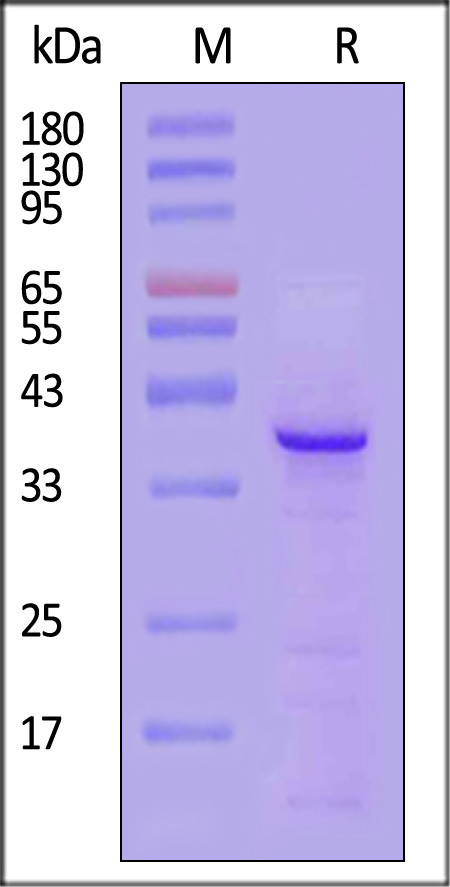
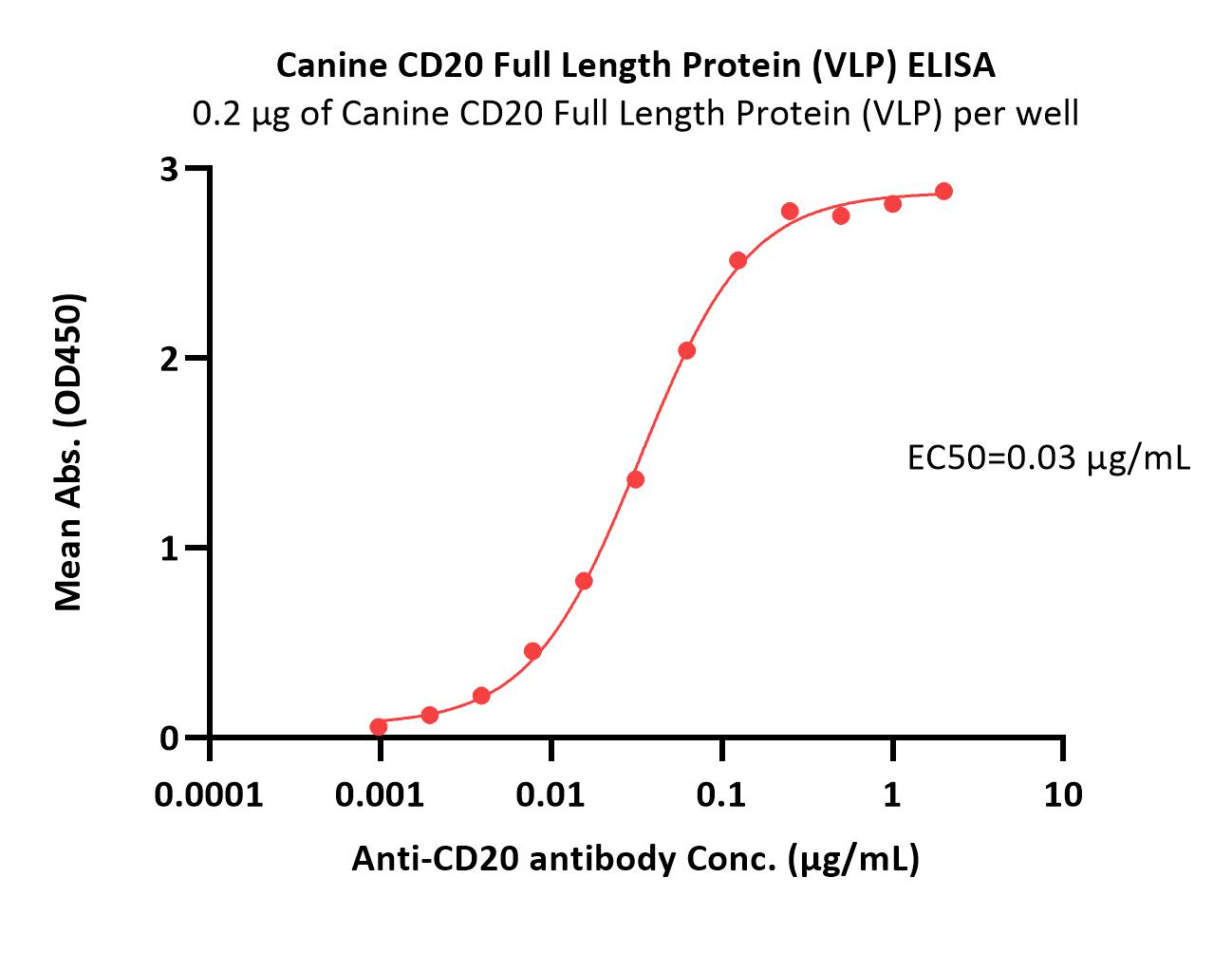
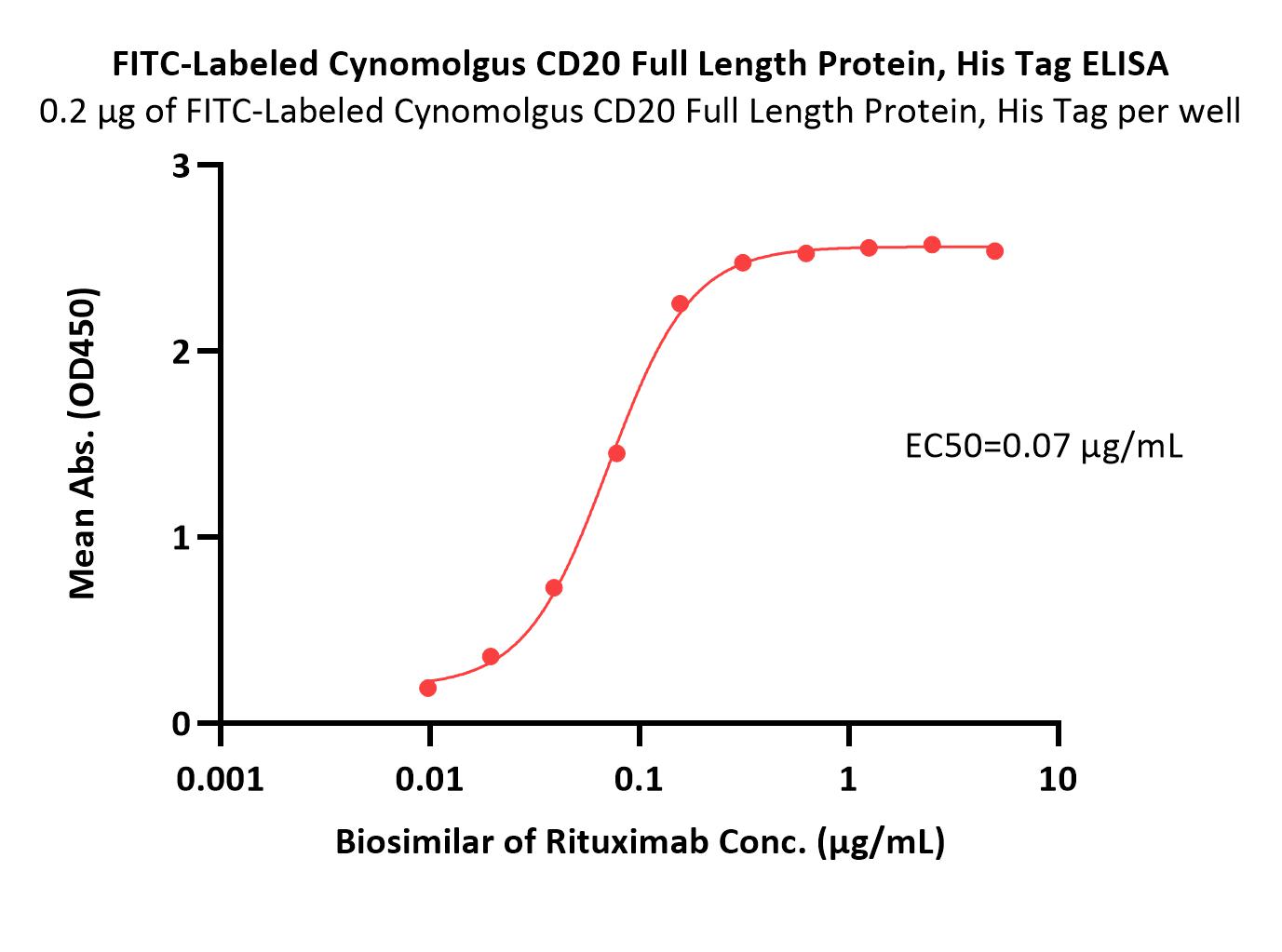
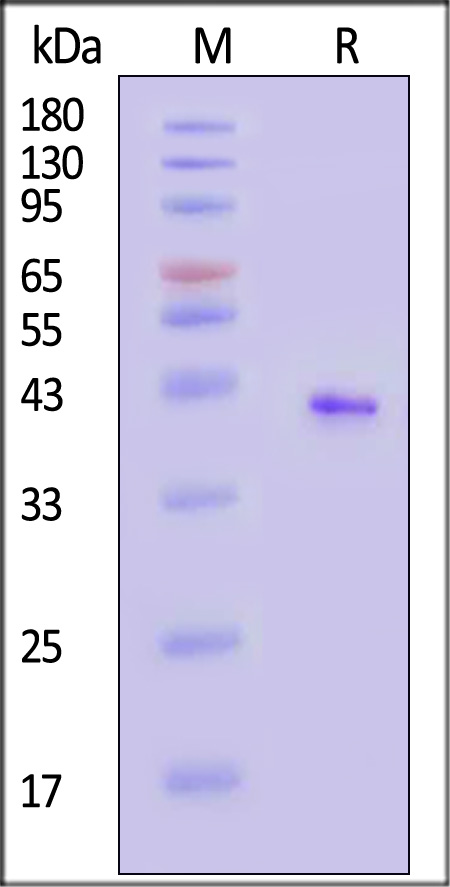
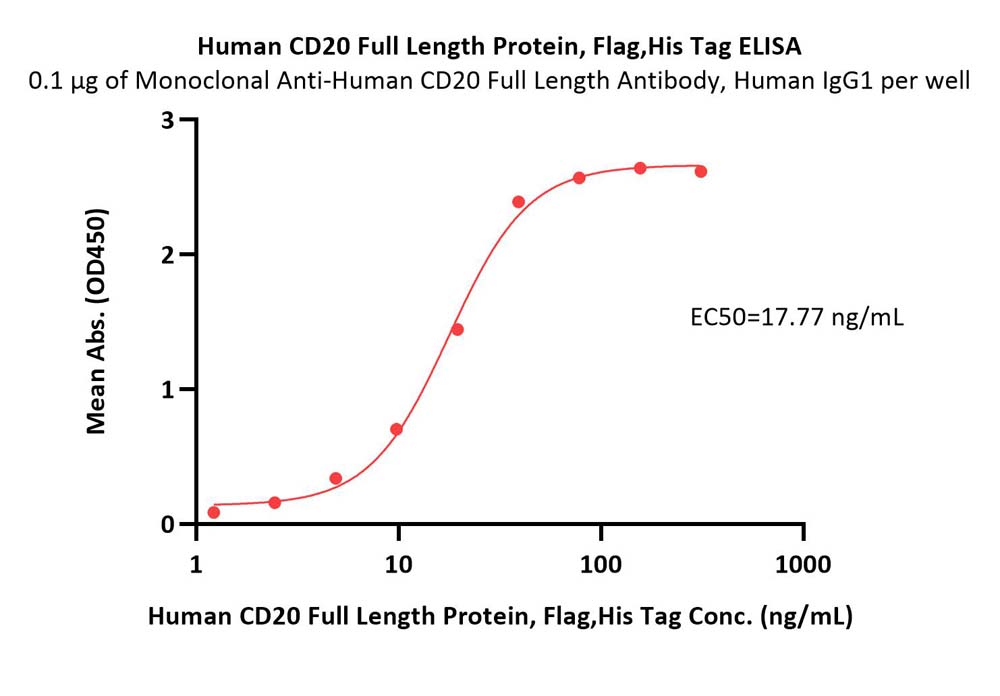
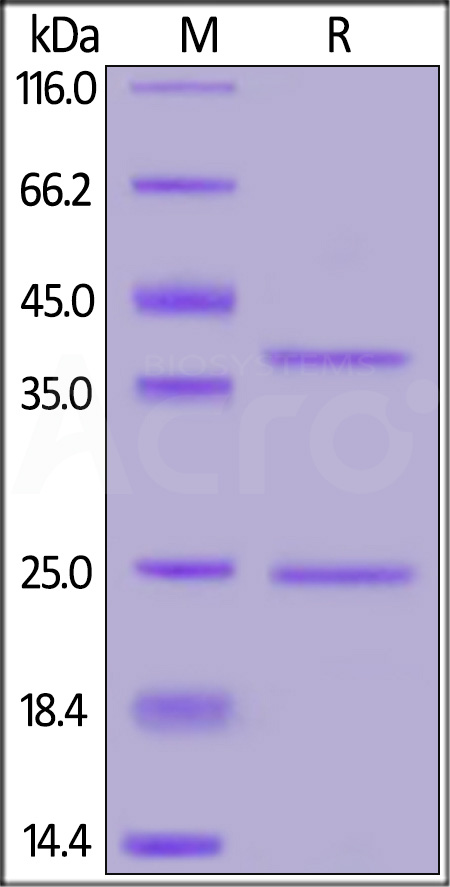
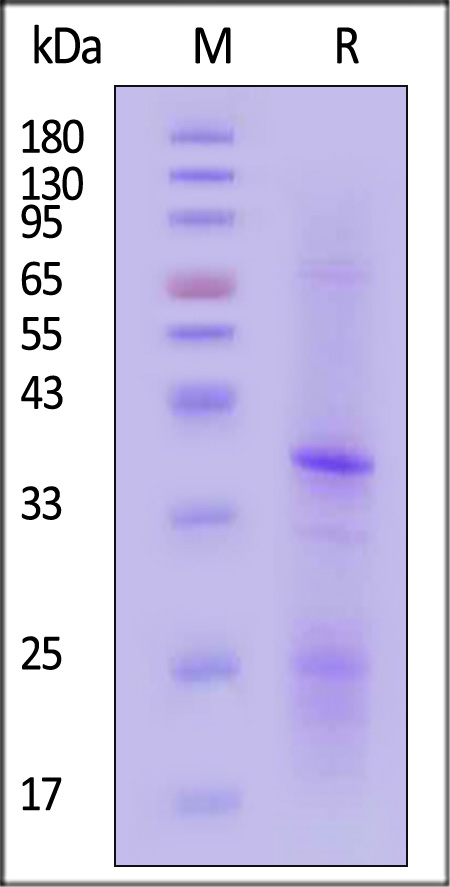
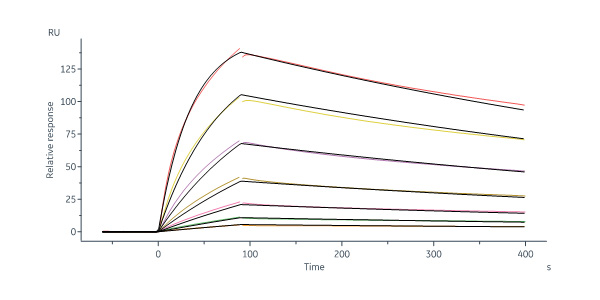
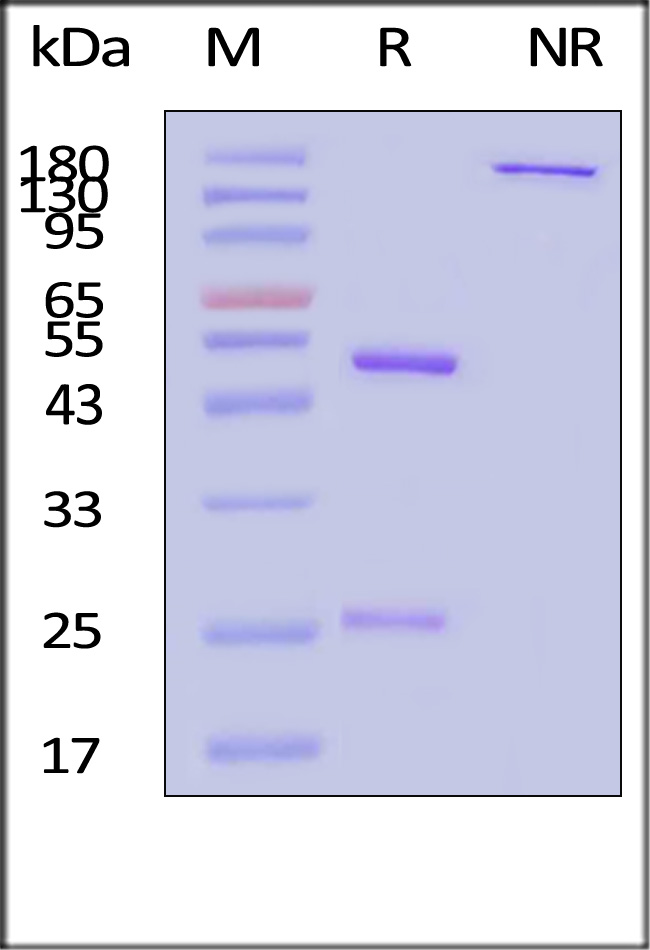
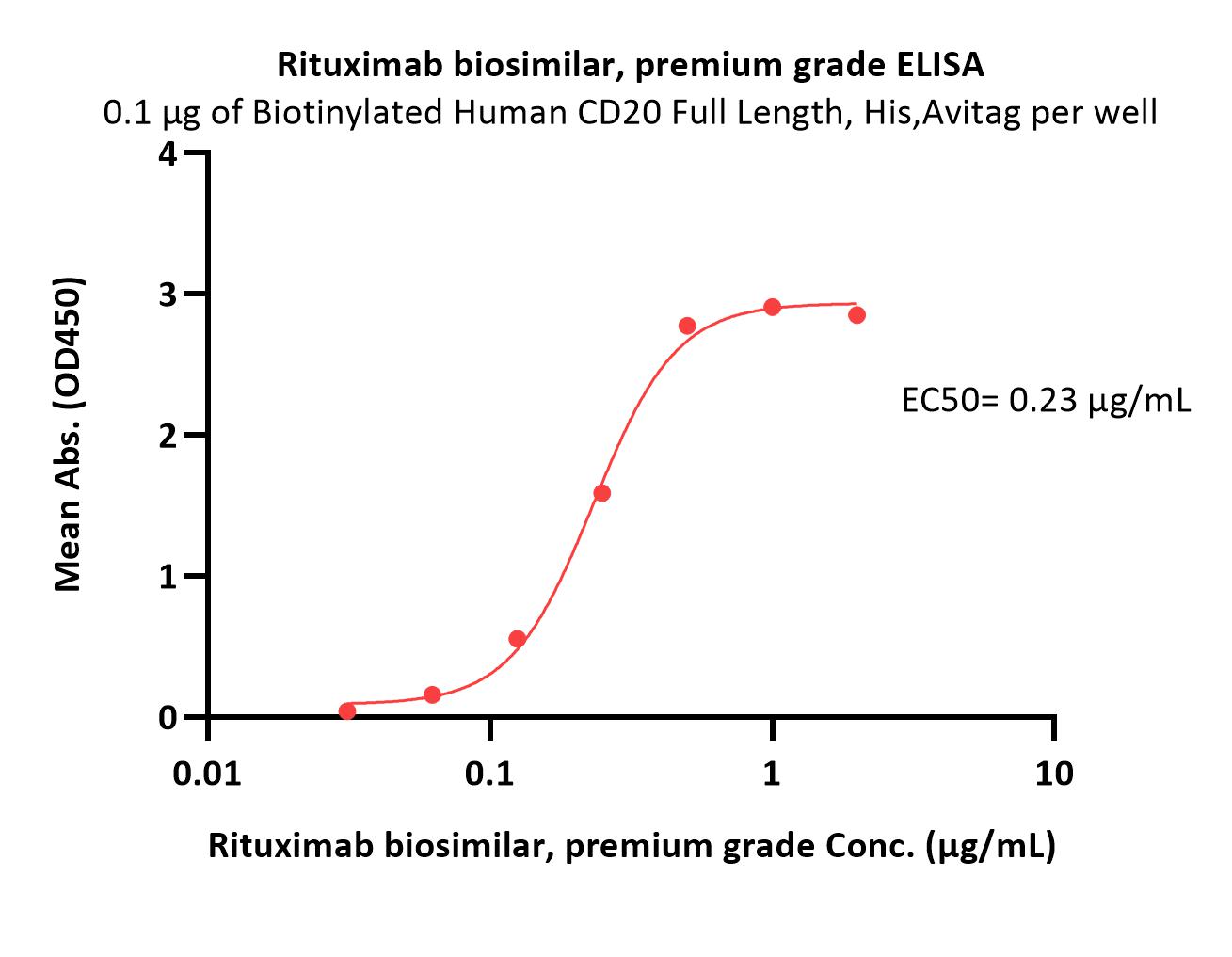
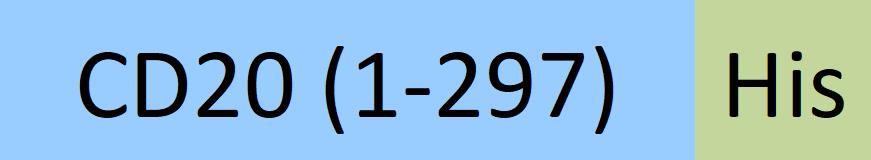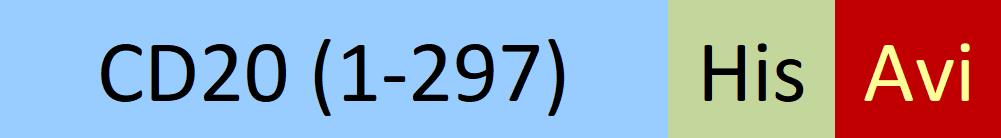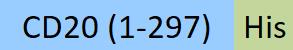CD20分子别名
MS4A1,CD20,MS4A-1
CD20分子背景
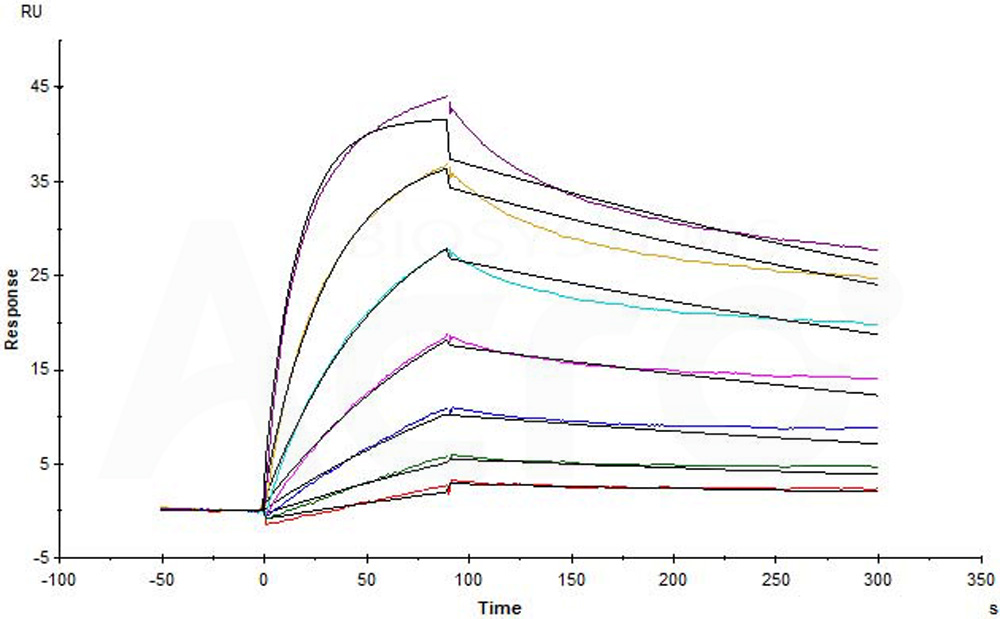
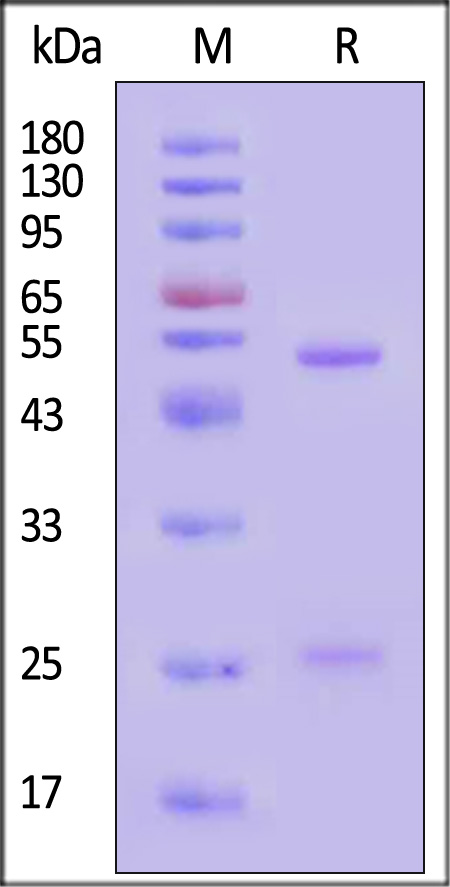
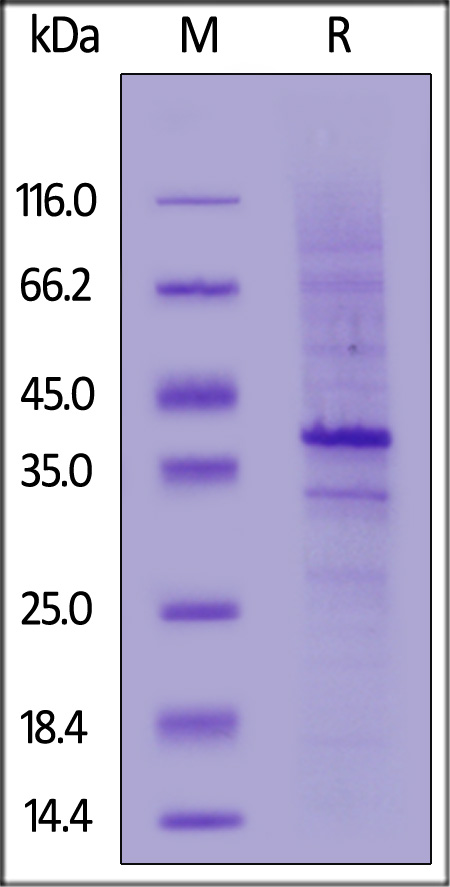
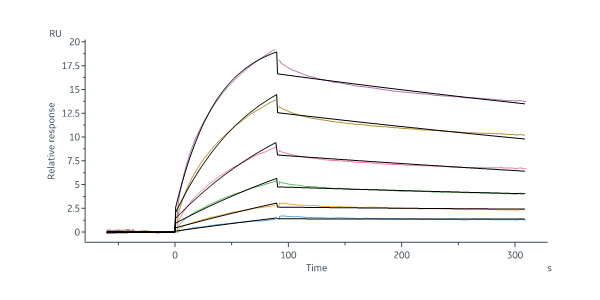
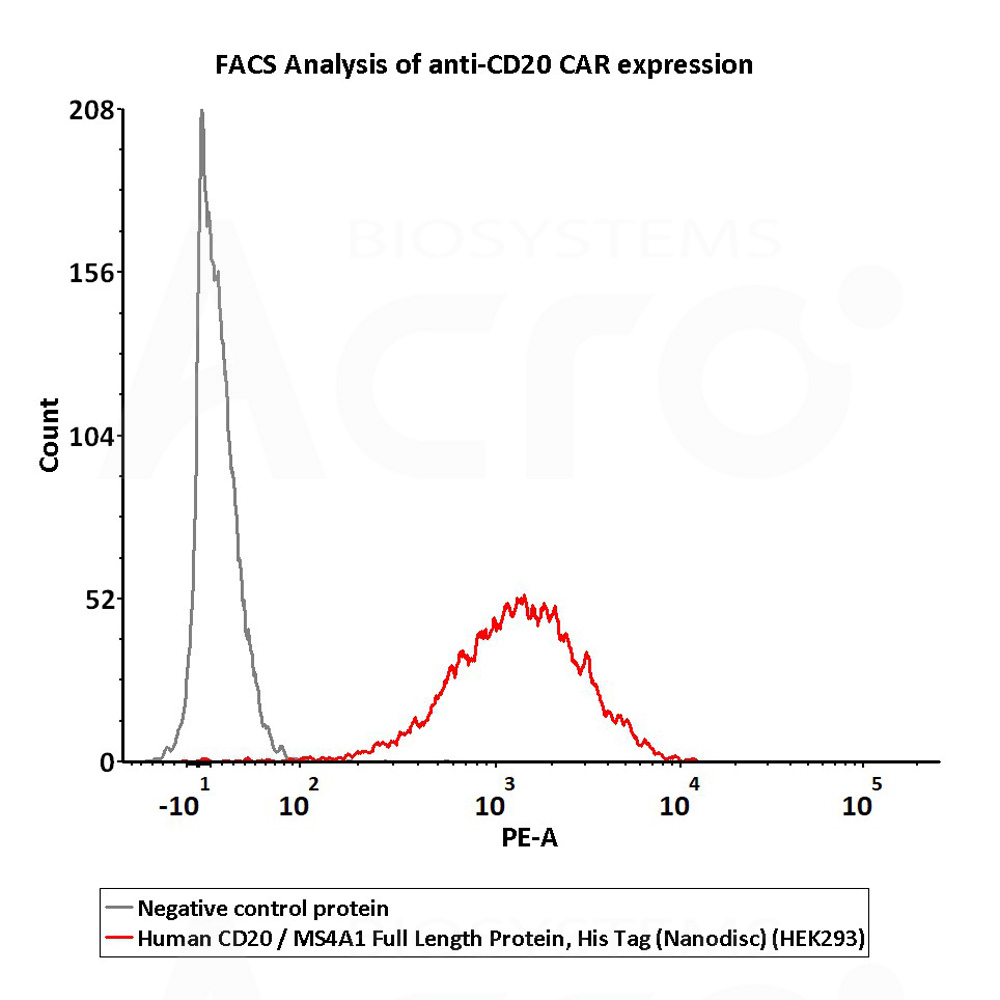
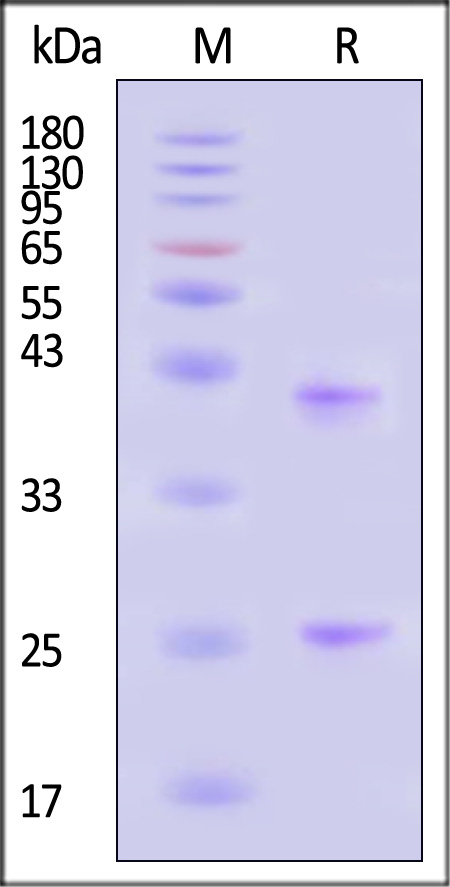
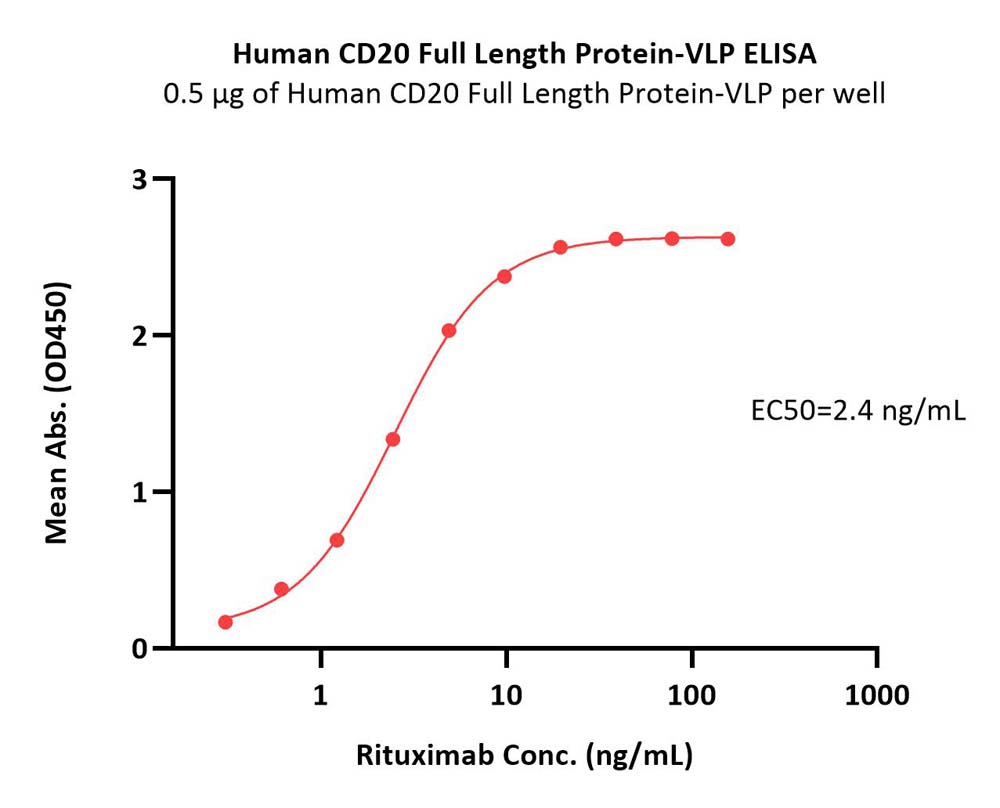
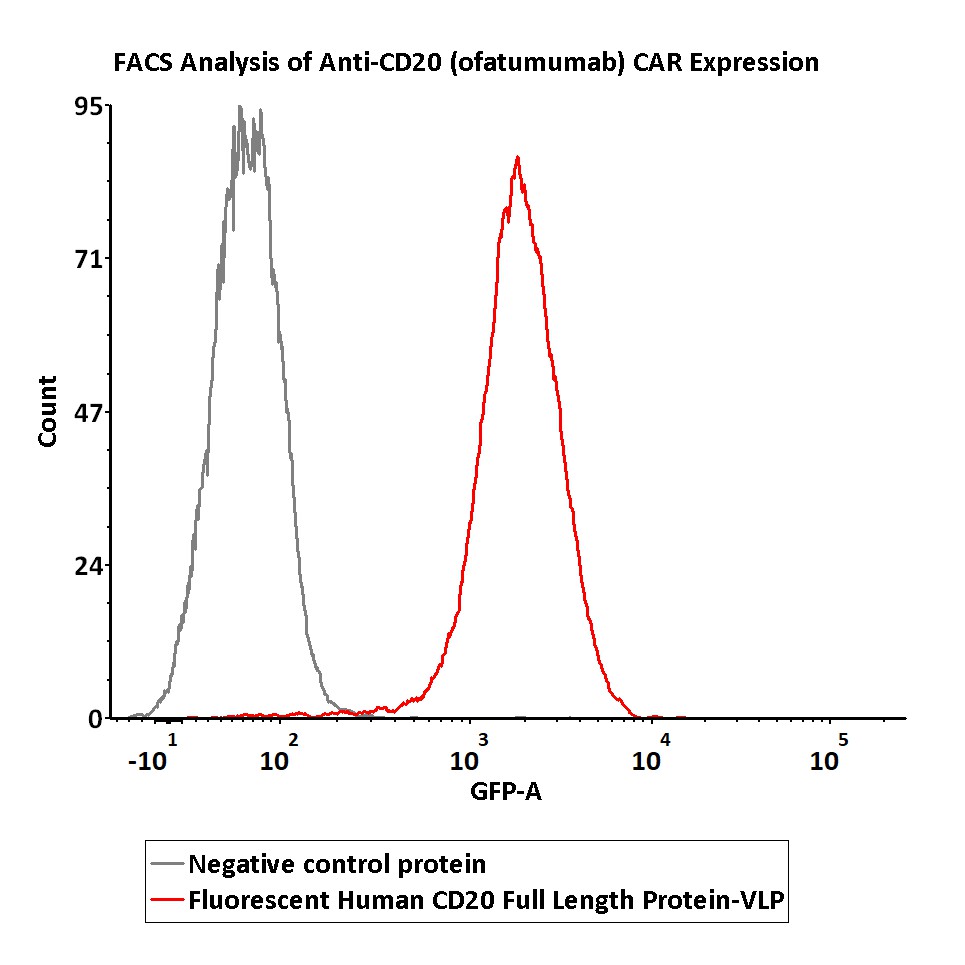
B-lymphocyte antigen CD20 is also known as B-lymphocyte surface antigen B1, Leukocyte surface antigen Leu-16, Membrane-spanning 4-domains subfamily A member 1 and MS4A1, is an activated-glycosylated phosphoprotein expressed on the surface of all B-cells beginning at the pro-B phase (CD45R+, CD117+) and progressively increasing in concentration until maturity. CD20 is expressed on all stages of B cell development except the first and last; it is present from late pro-B cells through memory cells, but not on either early pro-B cells or plasma blasts and plasma cells. It is found on B-cell lymphomas, hairy cell leukemia, B-cell chronic lymphocytic leukemia, and melanoma cancer stem cells. The protein has no known natural ligand and its function is to enable optimal B-cell immune response, specifically against T-independent antigens. It is suspected that it acts as a calcium channel in the cell membrane. CD20 / MS4A1 is the target of the monoclonal antibodies (mAb) rituximab, Ibritumomab tiuxetan, and tositumomab, which are all active agents in the treatment of all B cell lymphomas and leukemias. Defects in CD20 / MS4A1 are the cause of immunodeficiency common variable type 5 (CVID5); also called antibody deficiency due to CD20 defect. CVID5 is a primary immunodeficiency characterized by antibody deficiency, hypogammaglobulinemia, recurrent bacterial infections and an inability to mount an antibody response to antigen.






















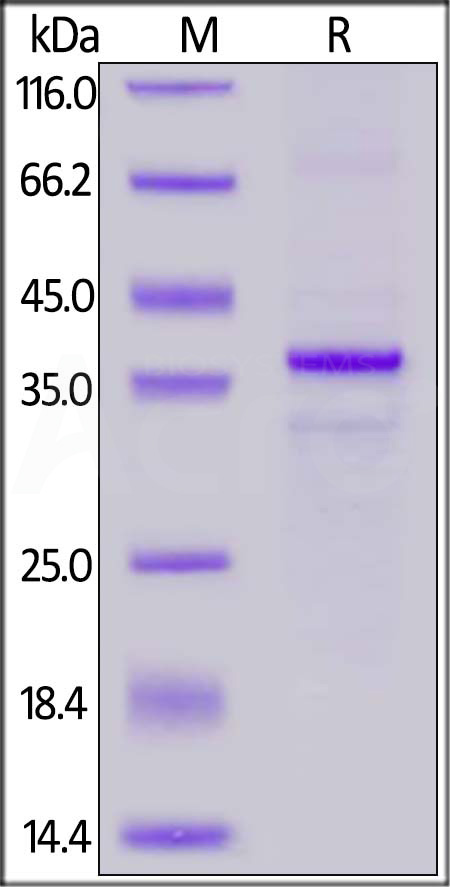
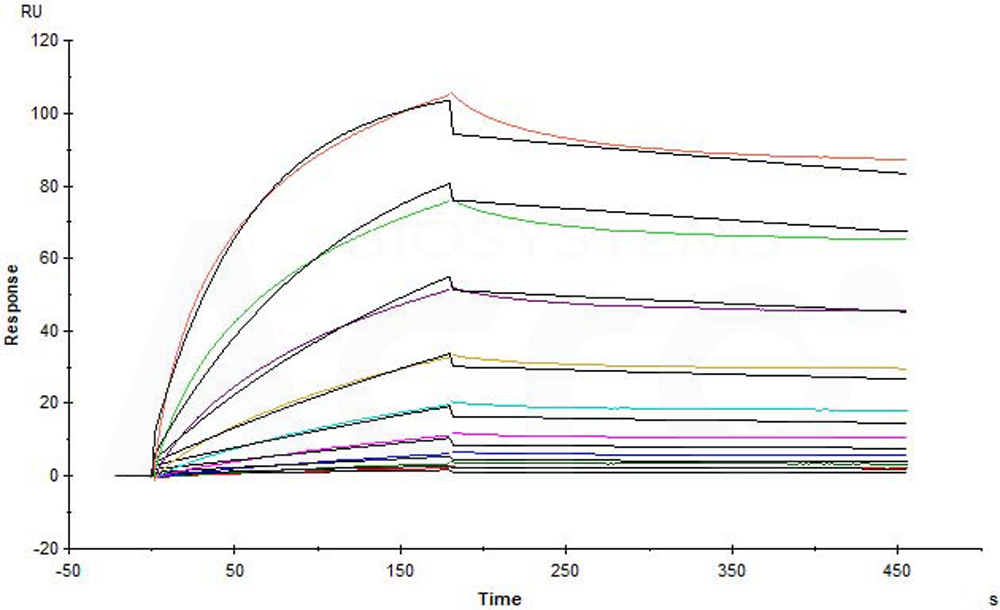
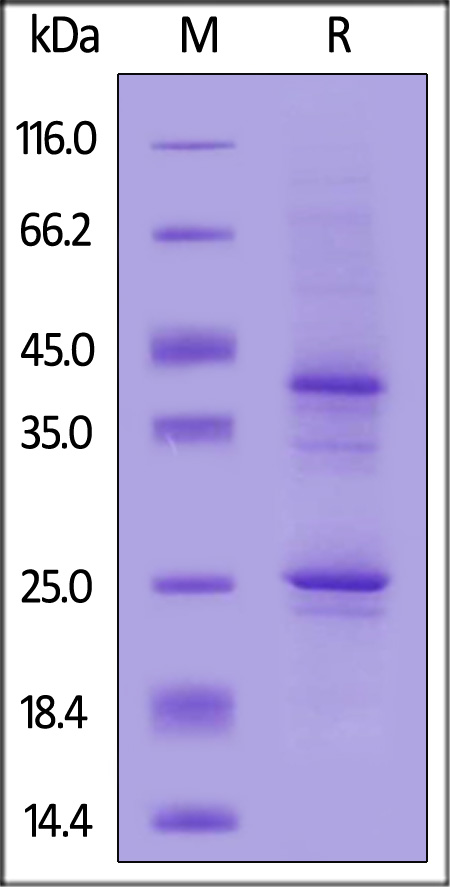
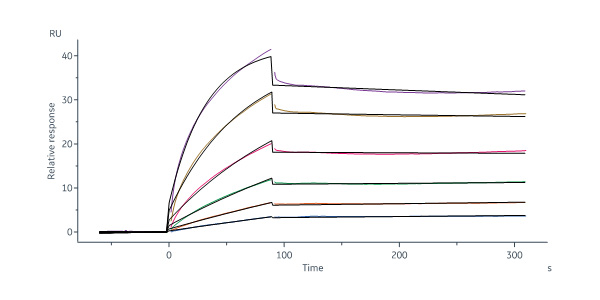
 Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!
Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!































 膜杰作
膜杰作 Star Staining
Star Staining