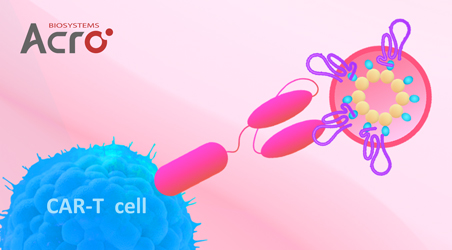
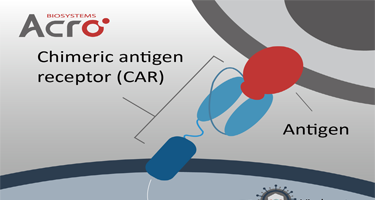
靶点特异性磁珠:助力CAR细胞疗法富集与体外分析
为满足市场需求,ACROBiosystems百普赛斯依托完善的抗原蛋白开发平台、磁珠偶联平台以及流式细胞术平台,成功开发了一系列ActiveMax®靶点特异性激活磁珠,经严格质量控制,产品具有无动物源、无菌、无支原体、超低内毒素等特性,支持CAR细胞的富集、体外分析等,适用于免疫细胞治疗相关研究和临床前工艺开发,且可定制GMP级别磁珠以应用于商业化生产的无缝衔接。
工程化免疫细胞疗法是肿瘤治疗领域的最新代表,尤其是嵌合抗原受体T细胞(CAR-T),在复发性或难治性多种血液系统癌症方面显示出显著疗效。由于CAR细胞是“活的药物”,其生产制备过程繁杂、质控要求严格,因此,迫切需要高质量的CAR细胞相关试剂/工具运用到药物开发中。研究显示,靶点抗原多聚物因其具有高靶点特异性、高精确性等特征在CAR细胞的富集分离、体外分析等多个场景广泛应用,尤其是基于磁珠偶联的特异性靶点多聚物展现出独特的应用优势。
CAR细胞的富集是细胞治疗药物开发中的常见操作,其对于CAR-T细胞输注产品的生产至关重要。在低水平CAR表达的细胞(低丰度和中丰度)这种困难的富集条件下,可采用特异性抗原偶联磁珠策略来进行CAR-T细胞富集,相对于四聚体,磁珠介导的十二聚体的细胞富集效率更高,可能是因为十二聚体比四聚体染色产生更高的荧光。这些结果表明,特异性靶点磁珠可用于富集低丰度CAR细胞或稀有CAR-T细胞。

利用特异性抗原偶联磁珠进行CAR-T细胞富集,Matter. 2021;4(12):3917-3940
对CAR-T细胞的表型、在各种肿瘤微环境(包括不同抗原密度)中的激活数量和质量以及由此产生的效应功能进行精确且标准化的体外评估,对于成功开发CAR-T疗法并安全地应用于临床至关重要。在CAR-T细胞药物临床使用前的体外分析中,特异性靶点偶联磁珠可精确控制磁珠表面抗原密度,与CAR-T细胞共孵育后,通过流式细胞术或ELISA测定等手段,可以定量检测CAR-T细胞的抗原特异性、剂量依赖性激活等指标,有助于建立标准化的CAR-T细胞体外测定方法。

使用包被了不同HER2抗原密度的磁珠与HER2 CAR-T细胞孵育后的相关体外分析测定
Front Immunol. 2022 Oct 20;13:994532.
为满足市场需求,ACROBiosystems百普赛斯依托完善的抗原蛋白开发平台、磁珠偶联平台以及流式细胞术平台,成功开发了一系列ActiveMax®靶点特异性激活磁珠,经严格质量控制,产品具有无动物源、无菌、无支原体、超低内毒素等特性,支持CAR细胞的富集、体外分析等,适用于免疫细胞治疗相关研究和临床前工艺开发,且可定制GMP级别磁珠以应用于商业化生产的无缝衔接。
-
高安全性:无菌、超低内毒素(<2EU/mg)
-
预偶联高质量蛋白,高批间一致性
-
支持细胞特异性激活、体外分析等
-
现货发售,稳定供货
我们的富集验证是在低CAR表达的CAR-T细胞上进行的。在初始染色群体中CAR细胞数很少(~0.1%),将设定理论含0.1%的CD19 CAR-T细胞与Human CD19-μBeads室温孵育1h,通过磁极富集磁珠CAR-T细胞混合体,流式检测其细胞表型(CD3, CD19)。结果显示在Beads : cells在2:4时, CAR-T细胞富集效果较佳。
注:CD19 CAR-T:1×107/mL
CD19-μBeads:2.5×106/mL;5×106/mL;1×107/mL


After positive selection with the ActiveMax Human CD19 ubeads at 2:4 (beads:ceels), the proportion of CAR cells that enriched cells were ≈150-fold higher than that of the initial population ~ 0.1%.
CD19 μBeads以2×10⁴ μBeads/孔(相当于约28.5ng偶联CD19蛋白)的密度接种于96孔板,并分别以效靶比(E:T)5:1,1:1,1:5与CD19 CAR-T细胞共培养24小时。单独培养的CD19 CAR-T细胞作为对照。随后,对活化标志物CD69、CD137进行检测分析CAR-T的激活情况。并对效应细胞因子IFN-γ 和细胞毒性分子(颗粒酶B、穿孔素)进行激活后的分泌评估。

CD19 CAR-T cells were stimulated with ActiveMax Human CD19 μBeads (MBS-C002) for 24h , and cells were harvested for evaluating CD69, CD137 expression by flow cytometry. The results showed that the proportion of CD69 and CD137 in CD19 CAR-T cells was significantly increased after CD19 μBeads stimulation.

CD19 CAR-T cells were stimulated with ActiveMax Human CD19 μBeads (MBS-C002) for 24h, and cell-free supernatants were harvested for evaluating IFN-γ secretion by flow cytometry. The results showed that CD19 CAR-T cells released significantly larger amounts of IFN-γ into the supernatants in response to CD19 μBeads.

CD19 CAR-T cells were stimulated with ActiveMax Human CD19 μBeads (MBS-C002) for 24h, and cell-free supernatants were harvested for evaluating IFN-γ secretion by ELISA. The results showed that CD19 CAR-T cells released significantly larger amounts of IFN-γ into the supernatants in response to CD19 μBeads.

CD19 CAR-T cells were stimulated with ActiveMax Human CD19 μBeads (MBS-C002) for 24h, and cell-free supernatants were harvested for evaluating Granzyme B, Perforin secretion by ELISA. The results showed that CD19 CAR-T cells released significantly larger amounts of Granzyme B, Perforin into the supernatants in response to CD19 μBeads.
|
货号 |
产品详情 |
|
ActiveMax® Human CD19 μBeads, premium grade (for cells) DMF |
|
|
ActiveMax® Human MSLN μBeads, premium grade (for cells) DMF |
|
|
ActiveMax® Human BCMA μBeads, premium grade (for cells) DMF |
|
|
ActiveMax® Human CD7 μBeads, premium grade (for cells) |
|
|
ActiveMax® Human Her2 μBeads, premium grade (for cells) DMF |
|
|
ActiveMax® Human CD22 μBeads, premium grade (for cells) DMF |
|
|
ActiveMax® Human CD229 μBeads, premium grade (for cells) |
|
|
ActiveMax® Human CEACAM-5 / CD66e μBeads, premium grade (for cells) DMF |
|
|
ActiveMax® Human CD19 μBeads (non magnetic), premium grade (for cells) |